mk
имиња во трошки


Silky sharks give birth to live young, providing placentally derived nutrition throughout the developmental process. In females, the oviducts are modified to form uteri, with only the right ovary being functional. The embryos develop in longitudinally oriented individual chambers, with their heads pointing anteriorly in the uterus. When silky sharks are born, they range in length from 70 to 75 cm. Juveniles rapidly grow an additional 25 to 35 cm by their first winter, which is thought to enhance their survival.
Development - Life Cycle: indeterminate growth
Like most large sharks, adult silky sharks have very few predators. They may occasionally encounter a killer whale (Orcinus orca) or another large shark that might pose a threat. Juveniles and smaller adults can also fall prey to larger, more mature sharks. Individuals in these smaller size classes often form small groups to avoid predation.
One of the few regular predators of silky sharks is humans. Silky sharks are known to follow schools of tuna and are often caught as a by-catch in tuna fisheries. They are also harvested by directed pelagic shark fisheries, and taken by recreational fisherman.
Known Predators:
Unlike most members of the genus Carcharhinus, the pectoral fins of this species are sickle-shaped. The first dorsal fin is relatively small, with a rounded apex, which originates behind the pectoral fins. The second dorsal fin is also very small, with a long trailing tip that almost reaches the precaudal pit, which is a notch on the dorsal side of the shark that is located where the caudal fin, or tail fin, begins. Silky sharks are the largest members of their genus, reaching up to 346 kg in mass and 3.5 m in length. Females grow to be much larger than males. Upon reaching maturity, female silky sharks range from 2.1 to 2.3 m (7 to 7.5 ft) in length whereas male silky sharks range from 1.8 to 2.1 m (6 to 7 ft).
Silky sharks get their name from the silky feel of their hide. Their skin, as in other shark species, is covered with dermal denticles. However, the unusually dense packing of these structures in this species makes their skin feel much softer to the touch than the rougher skin that is commonly associated with sharks. Another distinctive feature of silky sharks is the shape of their teeth. They have between 14 to 17 teeth on each side their upper jaws, and these teeth are notched or serrated rather than concave, which is the condition in most other species of sharks.
The dorsal coloration of this species can vary greatly, from a dark brown to a blue-grey color. The ventral surface is generally white, but in some individuals the ventral surface of the pelvic and pectoral fins can have darkly colored tips.
Range mass: 178 to 346 kg.
Range length: 1.8 to 3.5 m.
Average length: 2.5 m.
Other Physical Features: endothermic ; heterothermic ; bilateral symmetry
Sexual Dimorphism: female larger
The age of silky sharks can be determined by counting the number of growth rings that develop on their vertebrae, with each band representing approximately one year of life. Silky sharks live to be 23 years of age on average, and it is estimated that they can live up 25 years in the wild. There are no records of silky sharks being kept and raised in captivity.
Range lifespan
Status: wild: 25 (high) years.
Average lifespan
Status: wild: 23 years.
Average lifespan
Status: wild: 23 years.
Silky sharks are mostly found in the coastal and oceanic waters of tropical oceans, mainly at temperatures above 23°C. They primarily inhabit continental and insular shelves, but have also been found over deep water reefs and in open ocean, slope, and shallow, coastal water habitats. This species has been found at depths of up to 500 m, and records show that they have been seen in waters as shallow as 18 meters. It has been noted that this species has a wider latitudinal distribution along continental shelves compared to the open ocean or along insular shelves.
During various stages of the life cycle, silky sharks transition between different habitats. For the first few years of life, juveniles live in nursery grounds and lead a demersal or semi-pelagic lifestyle. As they grow older and reach an average young adult length of about 130 cm, they migrate offshore to deeper waters. At this stage, they often join and travel with large schools of pelagic fish such as tuna, ensuring a constant food supply. Adult silky sharks return seasonally to continental and insular shelf areas in order to feed and reproduce. However, they tend to spend most of their time in deeper waters.
Range depth: 500 to 18 m.
Average depth: 200 m.
Habitat Regions: temperate ; tropical ; saltwater or marine
Aquatic Biomes: pelagic ; reef ; coastal
Silky sharks are found throughout the Pacific, Atlantic, and Indian Oceans between 40°N latitude and 40°S latitude. They favor sub-tropical waters and are among the world’s most abundant shark species. They are highly migratory sharks, but have been known to concentrate in the Gulf of Aden, the Gulf of Mexico, and along the coast of southern Baja California. Those located in the Atlantic Ocean tend to move with the Gulf Stream and the migrations of tuna, their primary food source. Populations of silky sharks in both the Pacific and Indian Oceans also take part in migratory patterns by moving toward slightly higher latitudes during the summer months.
Biogeographic Regions: indian ocean (Native ); atlantic ocean (Native ); pacific ocean (Native )
Other Geographic Terms: cosmopolitan
Silky sharks are generalist carnivores and typically feed on various species of fish, squid, and pelagic crabs, including red crab (Pleuroncodes planipes), jumbo squid (Dosidicus gigas), and chub mackerel (Scomber japonicas). Young silky sharks primarily feed upon jumbo squid, while adult silky sharks consume more red crabs and chub mackerel. Additionally, yellowfin tuna (Thunnus albacares), albacore (Thunnus alalunga), mullet (Mugilidae species), and porcupine fish (Diodon holocanthus) have been found in the stomachs of silky sharks.
Variation in diet of silky sharks depends on the availability and abundance of prey. Other factors that affect their diet include the size and energy content of prey items, and seasonal changes in their availability. They primarily feed on schooling fish, most likely because of an increased likelihood of catching more prey, which reduces the amount of energy used in foraging. When food is limited, silky sharks act as opportunistic feeders, consuming a wide variety of prey from different habitats and depths in the open ocean. When food is abundant, they may be more selective in what they eat.
Animal Foods: fish; mollusks; aquatic crustaceans
Primary Diet: carnivore (Piscivore , Eats non-insect arthropods, Molluscivore )
Silky sharks are top-level predators, feeding at very high trophic levels. They feed on many species of fish and also serve as hosts to various parasites including isopods, copepods, and tapeworms. These parasites are commonly found in pelegic fish and in other members of the genus Carcharhinus.
Commensal/Parasitic Species:
Silky sharks have been the subject of many scientific studies surrounding the sensory biology of sharks. They are also among the most common bycatch species in the tuna fishing process, making up 70 to 80% of the pelagic longline catch off the coast of the Maldives and Sri Lanka. Many fishermen will remove their fins for sale in Asian markets, occasionally selling the meat and oils as well. Silky sharks are one of the most common sources of cleaned and dried shark jaws sold to tourists in tropical countries.
Positive Impacts: body parts are source of valuable material; research and education
Silky sharks can be dangerous to humans due to their large size and aggressive nature. They should be treated with extreme caution if encountered by divers, as they have been involved in documented attacks on humans. Such attacks are rare, however, as this species is typically found in the open ocean.
Negative Impacts: injures humans (bites or stings)
Silky sharks are considered near threatened on the IUCN Redlist and are vulnerable to over-fishing because of their long gestation period, low number of offspring, and slow growth rate. However, there has been very little sampling of silky shark populations in tropical waters. It is estimated that the population of silky sharks has decreased by 85% over the course of a 19 year period (1984-2005) and is continuing to decrease. These numbers are uncertain, however, due to the under-reporting of catch rates and lack of population monitoring. States and areas that allow fishing for this species have been encouraged to cooperate over its management to date no regulatory plans have been enacted.
US Federal List: no special status
CITES: no special status
State of Michigan List: no special status
There has been very little research conducted specifically on how silky sharks communicate and locate prey but, as with other shark species, they have several highly-developed senses. They have a superior sense of smell and can detect a single drop of blood in 100 L of water. They have paired nostrils beneath their snouts, which function as tunnels with two openings separated by a fleshy flap. As the shark swims forward, water flows over the olfactory glands, allowing the shark to “smell” the water. Silky sharks are also very sensitive to electrical frequencies and can accurately hear sounds 80 Hz and below, as well as sounds up to 800 or 1000 Hz. They can hear sounds that are imperceptible to the human ear such as the sounds of struggling prey, or the drumming of bony fish. It is thought that sharks have the ability to determine the direction a sound is coming from using their lateral line, or acousticolateralis system. This system is composed of small bundles of sensory cells called neuromasts which are located in pores along the head and body.
Silky sharks have been observed communicating using aggression displays, involving a raised head, arched back, and lowered tail. Males can also communicate by releasing pheromones into the water to attract females and ward off challenging males.
Communication Channels: visual ; chemical
Other Communication Modes: pheromones
Perception Channels: visual ; tactile ; acoustic ; vibrations ; chemical ; electric
Male silky sharks release pheromones; however, it is uncertain as to whether or not the pheromones are used to attract mates, ward off competition, mark territory, or some combination of the three. Additionally, studies have shown that no sexual segregation exists within silky shark populations. Pheromones do not play a role in determining social structure, meaning that silky sharks do not travel together solely for mating purposes. Rather, size appears to be the determing factor in social structure, with co-travelling generally being of the same size class.
Mating rituals of silky sharks, if they exist, are unknown. During the mating process, the male inserts his claspers into the female's cloaca, releasing sperm. Males mate with multiple females during a breeding season. In tropical waters, silky sharks do not have a set breeding season and mate year-round. Silky sharks located in the warm temperate waters of the Gulf of Mexico have a set breeding period during the summer months of June, July, and August.
Mating System: polygynous
Reproductive maturity is reached at 7 to 9 years of age and 2.1 to 2.3 m in length in females, 6 to 7 years and 1.8 to 2.1 m in males. Silky sharks in the Indian Ocean and in the Pacific Ocean achieve maturity at younger ages and at smaller sizes than silky sharks in other areas (approximately two years younger and 0.3 to 0.6 meters shorter). It is thought that the variation in size at maturity might be related to latitude, with sharks in tropical waters (areas of low latitude) tending to grow faster and mature at earlier stages of life. This may be due to warmer waters causing an increase in metabolism, thus speeding up growth rates, but the mechanism responsible is in need of additional research and confirmation.
In tropical waters, silky sharks breed year round, and in warm-temperate waters, such as the Gulf of Mexico, silky sharks breed only during the summer months (June, July, and August). They breed every two years and typically produce between two and fourteen live offspring per litter. The gestation period averages 12 months. Silky sharks are considered capable predators at birth.
Breeding interval: Silky sharks breed every two years.
Breeding season: In tropical waters, silky sharks breed year-round. In the warm-temperate waters of the Gulf of Mexico, silky sharks breed during the summer months (June, July, and August).
Range number of offspring: 2 to 14.
Average gestation period: 12 months.
Range age at sexual or reproductive maturity (female): 7 to 12 years.
Average age at sexual or reproductive maturity (female): 8 years.
Range age at sexual or reproductive maturity (male): 6 to 10 years.
Average age at sexual or reproductive maturity (male): 6.5 years.
Key Reproductive Features: iteroparous ; seasonal breeding ; year-round breeding ; gonochoric/gonochoristic/dioecious (sexes separate); sexual ; fertilization (Internal ); ovoviviparous
Female silky sharks provide continual nutrition to their developing young through the placenta. The young are also protected due to their development inside their much larger mother's body. Newborn silky sharks receive no additional parental care, as they are highly capable predators at birth. Given the patterns of reproduction known from other elasmobranch species, it is highly unlikely that males provide any investment during the 12 month gestation period.
Parental Investment: female parental care ; pre-hatching/birth (Provisioning: Female, Protecting: Female)
El tauró sedós (Carcharhinus falciformis) és un tauró que sol fer de 2 a 2,5 metres de llarg[1] però pot assolir els 3,30 m de longitud[2] i viure 25 anys.
És de distribució circumtropical, i a l'Atlàntic oriental se'l troba entre Madeira i Angola.[2] S'alimenta de peixos i en menor mesura de cefalòpodes i crancs. De vegades acompanya bancs de tonyina.[2]
És vivípar i cria cada any o cada dos anys, amb entre 6 i 12 cries a cada part després d'una gestació de 12 mesos.[3]
El tauró sedós (Carcharhinus falciformis) és un tauró que sol fer de 2 a 2,5 metres de llarg però pot assolir els 3,30 m de longitud i viure 25 anys.
És de distribució circumtropical, i a l'Atlàntic oriental se'l troba entre Madeira i Angola. S'alimenta de peixos i en menor mesura de cefalòpodes i crancs. De vegades acompanya bancs de tonyina.
És vivípar i cria cada any o cada dos anys, amb entre 6 i 12 cries a cada part després d'una gestació de 12 mesos.
Žralok hedvábný (Carcharhinus falciformis) je druh z čeledi modrounovitých, pojmenovaný podle svého jemného povrchu. Žije v tropických vodách celého světa, nejčastěji na kraji kontinentálních šelfů v hloubkách do padesáti metrů. Dorůstá obvykle délky 2,5 metru.
V roce 2007 změnil Mezinárodní svaz ochrany přírody jeho stupeň ohrožení z „málo dotčený“ na „téměř ohrožený“.
Žralok hedvábný (Carcharhinus falciformis) je druh z čeledi modrounovitých, pojmenovaný podle svého jemného povrchu. Žije v tropických vodách celého světa, nejčastěji na kraji kontinentálních šelfů v hloubkách do padesáti metrů. Dorůstá obvykle délky 2,5 metru.
V roce 2007 změnil Mezinárodní svaz ochrany přírody jeho stupeň ohrožení z „málo dotčený“ na „téměř ohrožený“.
Der Seidenhai (Carcharhinus falciformis) gehört zur Familie der Requiemhaie (Carcharhinidae) und wird von der IUCN als potenziell gefährdet eingestuft.[1]
Der Seidenhai wird 2 bis 3,3 m groß, mit grauer, braungrauer bis zu schwärzlicher Färbung, zum Bauch hin wird der Hai heller und weiß. Er ist ein großer und schlanker Hai mit einer mäßig langen, flachen und abgerundeten Schnauze, großen Augen, kleinem Kiefer und er besitzt spitze Zähne. Die erste Rückenflosse beginnt hinter dem freien Ende der Brustflosse (das typischste Merkmal). Die zweite Rückenflosse besitzt eine verlängerte Spitze.[2]
Der Seidenhai kommt weltweit in tropischen und subtropischen Regionen vor, sowohl in Küstennähe als auch ozeanisch:
Dieser Hai gehört zusammen mit dem Blauhai und dem Weißspitzen-Hochseehai zu den häufigsten großen Tieren der Hochsee. Die bevorzugte Wassertiefe liegt in den oberen 500 m, wo er vorwiegend Fische, aber auch Weichtiere jagt.
Der Seidenhai ist einer der drei meist gehandelten Haiarten der Welt, er ist meist Ziel der Fischer oder Beifang. Sein Fleisch wird zum Verzehr angeboten, wichtiger sind jedoch seine Flossen: die Flossen werden besonders in Hongkong gehandelt. Einigen Schätzungen nach, die bereits Beobachtungen von drei Haigenerationen umfassen, nimmt die Population des Seidenhais im Pazifik und im Atlantischen Ozean stetig ab. Sie zeigen aber auch eine Zunahme im Indischen Ozean. Es gibt aber Unsicherheiten über Fangquoten und Populationsveränderungen der Haie, man schätzt jedoch einen Rückgang der Art von 16 bis 22 Prozent über die letzten drei Generationen. Laut der FAO betrug der Fang 2014 ca. 4900 Tonnen und war vorwiegend in Sri Lanka gefischt. Aufgrund der vagen Daten kann man dem Fischfang nicht sicher die alleinige Schuld an der Populationsabnahme geben. Durch Umwelteinflüsse könnte diese Art auch an Bestand verlieren. Aufgrund des geschätzten Rückgangs der Population wird diese Art als potenziell gefährdet eingestuft.[1]
Der Seidenhai (Carcharhinus falciformis) gehört zur Familie der Requiemhaie (Carcharhinidae) und wird von der IUCN als potenziell gefährdet eingestuft.
Ο λείος καρχαρίας (επιστημονική ονομασία Carcharhinus falciformis - Καρχαρίνος ο δρεπανόμορφος) είναι καρχαρίας της οικογένειας των Καρχαρινιδών, γνωστός για τη λεία υφή του δέρματός του. Είναι ένας από τους πιο άφθονους πελαγικούς καρχαρίες, μαζί με τον γλαυκοκαρχαρία και τον ωκεάνιο λευκοπτέρυγο καρχαρία, και είναι το μεγάλο πελαγικό είδος με τον μεγαλύτερο πληθυσμό, τουλάχιστον δεκάδων εκατομμυρίων.[2] Βρίσκεται στα τροπικά νερά όλου του κόσμου.[3][4] Είναι πολύ κινητικός και μεταναστευτικός καρχαρίας και βρίσκεται συνήθως πάνω από την άκρη της ηπειρωτικής υφαλοκρηπίδας, σε βάθος 50 μέτρων. Έχει λεπτό, υδροδυναμικό σώμα και συνήθως φτάνει σε μήκος 2,5 μέτρων, αν και έχουν καταγραφεί καρχαρίες με μήκος 3,5 μέτρα.[5] Ξεχωρίζει από τους άλλους παρόμοιους καρχαρίες από το σχετικά μικρό πρώτο ραχιαίο πτερύγιο, το μικρό δεύτερο ραχιαίο πτερυγίο και τα μακριά, όμοια με δρεπάνια πτερύγια.
Με τη λεία συχνά να σπανίζει στο ωκεάνιο περιβάλλον του, ο λείος καρχαρίας είναι ένας γρήγορος, περίεργος και επίμονος κυνηγός. Τρέφεται κυρίως με οστεϊχθύς και κεφαλόποδα, και είναι γνωστό ότι οδηγούν τα ψάρια σε συμπιεσμένα κοπάδια[3] πριν αρχίσουν να περνούν από μέσα τους με ανοιχτό στόμα.[6] Αυτό το είδος ακολουθεί συχνά κοπάδια τόνου, ενός αγαπημένου θηράματος. Η αίσθηση της ακοής είναι ιδιαίτερα οξεία, επιτρέποντάς τους να εντοπίσουν τους χαμηλής συχνότητας ήχους που παράγονται από άλλα ζώα που διατρέφονται, και κατ' επέκταση, πηγές τροφής. Ο λείος καρχαρίας είναι ζωοτόκος, που σημαίνει ότι τα αναπτυσσόμενα έμβρυα που υφίστανται συνδέονται με πλακούντα με τη μητέρα τους. Υπάρχει σημαντική γεωγραφική διακύμανση στις λεπτομέρειες της ζωής του. Η αναπαραγωγή γίνεται όλο το χρόνο, εκτός από τον Κόλπο του Μεξικού, όπου ακολουθεί έναν εποχιακό κύκλο. Τα θηλυκά γεννούν μέχρι 16 νεογνά ετησίως ή ανά διετία.[1][3] Οι νεογέννητοι καρχαρίες περνούν τους πρώτους μήνες τους σε σχετικά προστατευμένους υφάλους στην εξωτερική υφαλοκρηπίδα, και αναπτύσσονται σημαντικά και πριν από τη μετάβαση στον ανοιχτό ωκεανό.
Το μεγάλο μέγεθος και τα κοφτερά δόντια του λείου καρχαρία τον καθιστούν ενδεχομένως επικίνδυνο, και έχει συμπεριφερθεί επιθετικά προς τους δύτες. Ωστόσο, οι επιθέσεις είναι σπάνιες, καθώς λίγοι άνθρωποι εισέρχονται στον ωκεάνιο βιότοπό του. Οι λείοι καρχαρίες εκτιμώνται για τα πτερύγιά τους, και σε μικρότερο βαθμό για το κρέας, το δέρμα τους, λάδι από το συκώτι, και τα σαγόνια. Λόγω της αφθονίας τους, αποτελούν ένα σημαντικό μέρος της εμπορικής και βιοτεχνικής αλιείας καρχαριών σε πολλές χώρες. Επιπλέον, η σύνδεση τους με τον τόνο έχει αποτέλεσμα πολλοί καρχαρίες να πιάνονται ως παρεμπίπτοντα αλιεύματα κατά την αλιεία τόνου. Αν και αναπαράγονται με αργούς ρυθμούς όπως και οι περισσότεροι άλλοι καρχαρίες, η ευρεία διάδοση και το μεγάλο μέγεθος του πληθυσμού του λείου καρχαρία θεωρούνταν ότι άμβλυνε τις πιέσεις προς το είδος. Ωστόσο, τα στοιχεία τώρα δείχνουν ότι οι αριθμοί των λείων καρχαριών μειώνονται σε όλο τον κόσμο, γεγονός που οδήγησε τη Διεθνή Ένωση για τη Διατήρηση της Φύσης (IUCN) να επανεκτιμήσει την κατάσταση διατήρησής του από ελαχίστης ανησυχίας σε Σχεδόν Απειλούμενο το 2007.[1]
Ο λείος καρχαρίας (επιστημονική ονομασία Carcharhinus falciformis - Καρχαρίνος ο δρεπανόμορφος) είναι καρχαρίας της οικογένειας των Καρχαρινιδών, γνωστός για τη λεία υφή του δέρματός του. Είναι ένας από τους πιο άφθονους πελαγικούς καρχαρίες, μαζί με τον γλαυκοκαρχαρία και τον ωκεάνιο λευκοπτέρυγο καρχαρία, και είναι το μεγάλο πελαγικό είδος με τον μεγαλύτερο πληθυσμό, τουλάχιστον δεκάδων εκατομμυρίων. Βρίσκεται στα τροπικά νερά όλου του κόσμου. Είναι πολύ κινητικός και μεταναστευτικός καρχαρίας και βρίσκεται συνήθως πάνω από την άκρη της ηπειρωτικής υφαλοκρηπίδας, σε βάθος 50 μέτρων. Έχει λεπτό, υδροδυναμικό σώμα και συνήθως φτάνει σε μήκος 2,5 μέτρων, αν και έχουν καταγραφεί καρχαρίες με μήκος 3,5 μέτρα. Ξεχωρίζει από τους άλλους παρόμοιους καρχαρίες από το σχετικά μικρό πρώτο ραχιαίο πτερύγιο, το μικρό δεύτερο ραχιαίο πτερυγίο και τα μακριά, όμοια με δρεπάνια πτερύγια.
Με τη λεία συχνά να σπανίζει στο ωκεάνιο περιβάλλον του, ο λείος καρχαρίας είναι ένας γρήγορος, περίεργος και επίμονος κυνηγός. Τρέφεται κυρίως με οστεϊχθύς και κεφαλόποδα, και είναι γνωστό ότι οδηγούν τα ψάρια σε συμπιεσμένα κοπάδια πριν αρχίσουν να περνούν από μέσα τους με ανοιχτό στόμα. Αυτό το είδος ακολουθεί συχνά κοπάδια τόνου, ενός αγαπημένου θηράματος. Η αίσθηση της ακοής είναι ιδιαίτερα οξεία, επιτρέποντάς τους να εντοπίσουν τους χαμηλής συχνότητας ήχους που παράγονται από άλλα ζώα που διατρέφονται, και κατ' επέκταση, πηγές τροφής. Ο λείος καρχαρίας είναι ζωοτόκος, που σημαίνει ότι τα αναπτυσσόμενα έμβρυα που υφίστανται συνδέονται με πλακούντα με τη μητέρα τους. Υπάρχει σημαντική γεωγραφική διακύμανση στις λεπτομέρειες της ζωής του. Η αναπαραγωγή γίνεται όλο το χρόνο, εκτός από τον Κόλπο του Μεξικού, όπου ακολουθεί έναν εποχιακό κύκλο. Τα θηλυκά γεννούν μέχρι 16 νεογνά ετησίως ή ανά διετία. Οι νεογέννητοι καρχαρίες περνούν τους πρώτους μήνες τους σε σχετικά προστατευμένους υφάλους στην εξωτερική υφαλοκρηπίδα, και αναπτύσσονται σημαντικά και πριν από τη μετάβαση στον ανοιχτό ωκεανό.
Το μεγάλο μέγεθος και τα κοφτερά δόντια του λείου καρχαρία τον καθιστούν ενδεχομένως επικίνδυνο, και έχει συμπεριφερθεί επιθετικά προς τους δύτες. Ωστόσο, οι επιθέσεις είναι σπάνιες, καθώς λίγοι άνθρωποι εισέρχονται στον ωκεάνιο βιότοπό του. Οι λείοι καρχαρίες εκτιμώνται για τα πτερύγιά τους, και σε μικρότερο βαθμό για το κρέας, το δέρμα τους, λάδι από το συκώτι, και τα σαγόνια. Λόγω της αφθονίας τους, αποτελούν ένα σημαντικό μέρος της εμπορικής και βιοτεχνικής αλιείας καρχαριών σε πολλές χώρες. Επιπλέον, η σύνδεση τους με τον τόνο έχει αποτέλεσμα πολλοί καρχαρίες να πιάνονται ως παρεμπίπτοντα αλιεύματα κατά την αλιεία τόνου. Αν και αναπαράγονται με αργούς ρυθμούς όπως και οι περισσότεροι άλλοι καρχαρίες, η ευρεία διάδοση και το μεγάλο μέγεθος του πληθυσμού του λείου καρχαρία θεωρούνταν ότι άμβλυνε τις πιέσεις προς το είδος. Ωστόσο, τα στοιχεία τώρα δείχνουν ότι οι αριθμοί των λείων καρχαριών μειώνονται σε όλο τον κόσμο, γεγονός που οδήγησε τη Διεθνή Ένωση για τη Διατήρηση της Φύσης (IUCN) να επανεκτιμήσει την κατάσταση διατήρησής του από ελαχίστης ανησυχίας σε Σχεδόν Απειλούμενο το 2007.
සිල්කි මෝරා යනු කාකරයිනිඩයි පවුලට (Carcharhinus falciformis) අයත් ක්ෂීරපායී මෝරුන් විශේෂයකි.සමෙහි ස්වභාවය අනුව ඔවුන් මේ ලෙස නම් කර ඇත.නිවර්තන කලාපීය සාගර වල නොගැඹුරු මුහුදේ මෙම මෝරුන් විශේෂය සුලබ වශයෙන් දක්නට ඇත.ඉතා චංචල ස්වභාවයක් ඇති මෙම මෝරා මහද්වීපික තටකයට මීටර් 50ක් (අඩි 164ක්) පමණ ගැඹුරු මුහුදේ දී දැක ගත හැකිය. අනාකූල සිහින් සිරුරකට හිමිකම් කියන මෙම මත්ස්යයා සාමාන්යයෙන් මීටර් 2.5ක් (අඩි 8යි අඟල් 2යි ) පමණ වර්ධනය වේ.සිරුර පසුපස ඇති වක්රාකාර දාරයක් සහිත කුඩා පෘෂ්ඨීය වරල නිසා මොවුන් අනෙක්කුත් මෝරන් අතරින් විශේෂ කොට හදුනා ගත හැක. එමෙන්ම නිදහස් ලෙස විහිදී ගිය දෑකැත්තක හැඩැති ලය වරල් යුගලයක්ද මෙම මත්සයාට ඇත.ඒවා මතුපිට ලෝකඩමය අළු පැහැයක් ඇති අතර යට ප්රදේශය සුදු පැහැයක් ගනී.
මොවුන් වෙසෙන සාගරික කලාපයේ ගොදුරු හිඟ වූ විට මොවුන් ඉතා වේගවත් මුරණ්ඩු ස්වභාවයකින් යුතු දඩයක්කාරයකු ලෙස හැසිරේ.මෙම මෝරු විශේෂය ප්රධාන වශයෙන් කටු සහිත මසුන් සහ සෙෆලපොඩ් නැමැති මසුන් විශේෂය ආහාරය ලෙස ලබා ගනී.තම මුඛය පුළුල් ලෙස විවෘත කරමින් කුඩා මසුන් රෑනක් එකවර හඹා ගොස් දරුණු ප්රහාරයක් එල්ල කිරිම සිදු කරයි.එමෙන්ම මොවුන් බලයා,කෙළවල්ලා වැනි මසුන්ගේ ඉව ඔස්සේ හඹා ගොස් ගොදුරු කර ගැනීමටද කැමත්තක් දක්වයි.ශ්රවණ සන්වේදනය ඉතාම තියුණු වන මෙම සත්වයාට තමන් ගොදුරු කර ගන්නා කුඩා වර්ගයේ මාළුන් විසින් ඇති කරන අඩු සංඛ්යාත හඩවල් ඔස්සේ යමින් ආහාර ප්රභව සොයා ගැනීම ඉතා පහසුවෙන් සිදු කරයි.
සිල්කි මෝරා ජලාබුජ සත්වයෙකි.එනම් කළලය පෝෂණය වන්නේ කළල බන්ධනය මගින් මවට සම්බන්ධ වීමෙනි.මෙය ඔවුන්ගේ ජීවිත කාලයේ එක් විශේෂ අවස්ථාවකි.මෙම සතුන්ගේ ප්රජනන ක්රියාවලිය ඍතු චක්රය අනුව සිදු වේ. මෙක්සිකෝවේ ගල්ෆ් කලාපයේ වෙසෙන ගැහැණු සතුන් හැර අනෙකුත් සාගර කලාප වල ජීවත් වන ගැහැණු සත්ත්වයා ප්රජනනයේදී වාර්ෂිකව හෝ ද්විවාර්ෂිකව පැටවුන් 16 දක්වා පමණ බිහි කරයි.අලුතින් බිහි වන පැටවුන් ගැඹුරු මුහුදෙහි ජීවත් වීමට තරම් වැඩෙන තෙක් මුල් මාස කිහිපය ගොඩබිමට ඈතින් ඇති ගල්පර ආශ්රිතව ගැවසෙමින් ජීවත් වේ.
සමහර අවස්ථා වලදී කිමිදුම් කරුවන්ටද ආක්රමණශීලී ප්රහාර එල්ල කරන මෙම මත්සයා සතු විශාල කැපෙන සුළු දත් නිසා ඔවුන්ට තවත් භයානක පෙනුමක් ලැබී ඇත.කෙසේ වුවද මිනිසුන් ඔවුන්ගේ වාසස්ථාන වලට ඇතුල් වූ අවස්ථා කිහිපයකදී හැර අනෙකුත් අවස්ථා වල එල්ල කරන ලද ප්රහාර දුර්ලභ වේ. සිල්කි මෝරාගේ වරල් ඉතා වටිනාකමින් වැඩි අතර සම,මෝර තෙල්,හකු හා මස්ද ලබා ගනී.මොවුන් ඉතා සුලභ බැවින් බොහෝ රටවල් වල මත්ස්ය කර්මාන්තයේ ප්රධාන සංරචකයක් ලෙස වැඩි වාණිජ වටිනාකමක් හිමි කර ගනී.තවද සිල්කි මෝරා බලයා,කෙලවළ්ලා වැනි මාළු විශේෂ සමඟ නිරන්තරයෙන් ගැවසෙන නිසා බලයන් කෙලවළ්ලන් ඇල්ලීම සිදු කරන බොහෝ අවස්ථා වල අහඹු ලෙස මෝරුන්ද දැල් වලට හසු වීම සිදු වේ.අනෙකුත් මෝරුන්ගේ මෙන්ම ප්රජනන ක්රියාවලිය සෙමෙන් සිදු වුවද සිල්කි මෝරා බොහෝ ප්රදේශයක පැතිරී ගිය සාමාන්ය ගහණයකින් යුතු මෝරුන් විශේෂයකි.නමුත් පසුගිය කාල වකවානුව තුල මත්ස්ය කර්මාන්තය හේතුවෙන් ඔවුන්ගේ ගහණය අඩු වී යන බවට ගණන් බලා ඇත. 2007 වසරේදී International Union for Conservation of Nature (IUCN) මගින් නිකුත් කර ඇති සංඛ්යා ලේඛන අනුව මෙම මෝරුන්ගේ සංරක්ෂණ තත්වය අවම වැදගත් සිට තර්ජනයට ලක්වීමට ආසන්න ලෙස ප්රකාශයට පත් කර ඇත.
සිල්කි මෝරා කාකරියස් ෆැල්සිෆෝමිස් ( Carcharias (Prionodon) falciformis ) කාණ්ඩයට එක් කරමින් ඔවුන් පිළිබද ප්රථම අර්ථ දැක්වීම සිදු කරනු ලැබුයේ ජර්මානු විද්යාඥයන් දෙදෙනෙකු වූ ජොහැන්ස් මුලර් හා ජකොබ් හෙන්ලේ විසිනි. මොවුන් දෙදෙනාගේ නිදර්ශනය වූයේ සෙන්ටි මීටර් 53ක් (අඟල් 21)ක් පමණ වූ භ්රෑණයකි. වෙනත් මෝරුන් විශේෂයක් ලෙස හදුනාගනු ලැබ තිබූ වැඩුණු සිල්කි මෝරා කාකරයිනස් ෆැල්සිෆෝමිස් ( Carcharhinus falciformis ) ලෙස නිවැරදිව හදුනා ගනු ලැබුවේ 1943 දී හෙන්රි බිග්ලෝ,විලියම් ස්කෝර්ඩර් හා ස්ටුවර්ට් ස්ප්රින්ගර් විසිනි. ෆැල්සිෆෝමිස් යනු දෑකැති හැඩයට ලතින් භාෂාවේ ඇති විශේෂණ පදයකි. එය මෙම මත්සයාගේ පෘෂ්ඨීය හා ලය වරල් හා සම්බන්ධ වන්නකි.සිල්කි මෝරා යන පොදු නම ඔවුන්ට ලැබී ඇත්තේ අනෙක් මෝරුන්ගේ සම සමඟ සංසන්දනය කල විට ඔවුන්ගේ සම ඉතා කදිම ලෙස කුඩා කොරපොතු වලින් වැසී මෘදු ස්වභාවයක් පැවතීම නිසාවෙනි.
සිල්කි මෝරාගේ පොසිල දතක් උතුරු කැරොලිනාවෙන් හමු වී ඇත.එය කාල නිර්ණයෙන් වසර 12,000ක් පමණ පැරණි බව සොයා ගෙන ඇත. සිල්කි මෝරාගේ මූලික පරිණාමය අවිනිශ්චිත වූවකි.1982 ප්රධාන ශරීරාංග සැලකීමෙන් ජැක් ගැරික් නම් විද්යඥයා බ්ලැක් ස්පොට් මෝරා ඔවුන්ගේ ආසන්නතම නෑදෑයෙක් ලෙස හදුනා ගැනිනි. 1988දී ලෙනාර්ඩ් කම්පැග්නෝ සිල්කි මෝරා තාවකාලික වශයෙන් බ්ලැක් නෝස් මෝරා,බ්ලැක්ටිප් රීෆ් මෝරා,නර්වස් මෝරා,කොපර් මෝරා,රාත්රී මෝරා ඇතුලත් කාණ්ඩයට ඇතුලත් කරන ලදී. 1992දී ගැවින් නේයිලර් සිල්කි මෝරාගේ වංශ ප්රවේණිය පිළිබද එන්සයිම මත පදනම් වෙමින් කල විශ්ලේෂණයකදී නැමුමක් සහිත ළය වරල් ඇති විශාල මෝරුන්ගේ කාණ්ඩයට අයත් බව සොයා ගන්නා ලදී.මෙම කාණ්ඩයේ එක් ශාඛාවකට සැන්ඩ්බාර් මෝරා,,විශාල නාසයක් ඇති මෝරා අයත් වන අතර සිල්කි මෝරා ප්රධාන ශාඛාවට අයත් වේ.මෙම කොටසට කැරිබියානු ගල්පර මෝරා,ගැලපෙගෝස් මෝරා,සාගරික වයිට්ටිප් මෝරා,ඩස්කි මෝරා,නිල් මෝරා ද අයත් වේ. රයිබසෝමීය ඩී.එන්.ඒ විශ්ලේෂණයකින් පසු මින් ඩොසේයි ඇබ්කලට් විසින් සිල්කි,නිල්,විශාල නාසයක් ඇති යන මෝරුන් විශේෂ 3 අතර සමීප සමානතා ඇති බව තහවුරු කරන ලදී.
සිල්කි මෝරා සෙල්සියස් අංශක 23 (ෆැරන්හයිට් අංශක 73) පමණ උෂ්ණත්වයක් ඇති සාගරික කලාපයේ ජීවත් වේ.අත්ලාන්තික් සාගරය ආශ්රිතව ගල්ෆ් මුහුදු තීරය,මෙක්සිකෝව හා කැරිබියානු මුහුදු තීරයෙන් මොවුන් සොයා ගත හැකිය.ඉන්දියන් මුහුද,බටහිර ඕස්ට්රේලියාවේ මොසැම්බික් රතු මුහුදු, පර්සියානු ගල්ෆ් මුහුදු තීරයෙන්ද දකුණු චීනය,ජපානය ආසන්න මුහුදු තීරයේද දකුණු බාජා කැලිෆෝනියා හා ගල්ෆ් කැලිෆෝනියා හා දකුණුදිග ඕස්ට්රේලියාවේ සිට උතුරු නවසීලන්තය සිට උතුරු චිලී දෙසටද මොවුන්ගේ වාසස්ථාන ව්යාප්ත වේ. සිල්කි මෝරුන් විශේෂ 4ක් ලෝකය පුරා සාගර ප්රදේශ වල පැතිර ඇත.වයඹ දිග අත්ලාන්තික් සාගරයේ බටහිර හා මධ්යම පැසිෆික් සාගරයේ නැගෙනහිර පැසිෆික් සාගරයේ හා ඉන්දියන් සාගරයේ ඔවුන් වෙසෙයි.
උෂ්ණ කලාපීය නැගෙනහිර පැසිෆික් හා උතුරු ගල්ෆ් මෙක්සිකෝවේ වෙසෙන සිල්කි මෝරුන්ගේ ගමන් මාර්ග අධ්යනයෙන් සාමාන්යයෙන් සිල්කි මෝරා මුහුදු මතුපිට සිට මීටර් 200(අඩි 660) ගැඹුරෙහි ජීවත් වන බව සොයා ගෙන ඇත.නමුත් ඔවුන්ට මීටර් 500 (අඩි 1600) හෝ ඊට වැඩි ප්රමණයක් දක්වා ගැඹුරට කිමිදීමටද හැකියාවක් ඇත .සිල්කි මෝරා තම වේලාවෙන් 99% ගත කරනුයේ මුහුදු මතුපිට සිට මීටර් 50 දක්වා වූ නොගැඹුරු මුහුදෙහිය.එමෙන්ම රාත්රිය දහවල ගැන අවධානයක් නොදක්වන මෙම සතුන් ඔවුන් මුහුදෙහි සැරිසරන වේලාවෙන් 80% - 85% ගතකරනුයේ උෂ්ණත්වය සෙල්සියස් අංශක 26 - 30 (ෆැරන්හයිට් අංශක 79 - 86) වන සාගර ජලයෙහිය.
මෙම සත්ත්ව විශේෂය දිවයින් ආශ්රිත ගල්පර සහිත ගැඹුරු මුහුදු තීරයට විශේෂ ලැදියාවක් දක්වයි.ඔවුන් උතුරු,දකුණු මහද්වීපික මායිම් අතර සැරිසැරීම සිදු කරයි.හදිසි අවස්ථාවන් වල මීටර් 18ක්(අඩි 59)තරම් නොගැඹුරු මුහුදු කලාප වලට පැමිණීමෙන් මොවුන් අනතුරුදායක අවස්ථාවන්ටද මුහුණ දේ.සිල්කි මෝරා ඉතා චංචල වෙනත් ප්රදේශ වලට සංක්රමණය වීමට කැමති සත්ත්වයෙකි.ඔවුන් පිළිබද අධ්යනයෙන් සාමාන්ය මෝරෙක් දිනකට කිලෝ මීටර් 60ක්(සැතපුම් 37ක්) පමණ පිහිනීම සිදු කරයි.එමෙන්ම ඔවුන් චලනය වන වපසරිය කිලෝ මීටර් 1339ක් (සැතපුම් 832)පමණ වේ.වැඩුණු සිල්කි මෝරා කුඩා පැටවුන්ට සාපේක්ෂව වැඩි දුරක් චලනය වේ. පැසිෆික් මුහුදේ හෝ වෙනත් ප්රදේශ වල ජීවත් වන මොවුන් ග්රීෂ්ම ඍතුවේදී සමක සීමාවෙන් තරමක් ඔබ්බට වන සාගර කලාප වලට ගමන් කරයි.
නාස් පුඩු වලට ඉදිරියෙන් වටකුරු ස්වභාවයක් උසුලන උලක් හැඩැති ශරීරාංගයක් ඇති සිල්කි මෝරා සුමට සිහින් දිග ශරීරයක් ඇති මත්ස්යයෙකි.වක්රාකාර මධ්යම ප්රමාණයේ ඇස් දෙක විනිවිද පෙනෙන (තුන් වන ඇසි පිය ලෙස හදුන්වන ) පටලයකින් සමන්විත වී ඇත.එමගින් ඇසට ආරක්ෂාව සැපයේ.මුඛය දෙපස සිහින් ලෙස රැලි ගැසීමක් දක්නට ඇත. උඩු හනුවෙහි හෝ යටි හනුවෙහි පිළිවෙලින් 14 - 16 හා 13 - 17 ප්රමාණයෙන් දත් පිහිටා ඇත.(මූලිකවම දත් 15 බැගින් හනු දෙකෙහිම ) උඩ පිහිටි දත් දැඩි ශක්තිමත් ත්රිකෝණාකාර,පිටුපසින් උල් හැඩයක් ගනී.එම දත් මැදදී කෙලින් ඇති අතර බොහෝ විට පැති දිශා වලට ආනතියක් දරයි.පහළ දත් පටු,කෙලින් ඇති සිනිදු මතුපිටක් සහිත වේ. කරමල් යුගල 5ක්ද මොවුන් සතුවේ.
පෘෂ්ඨීය හා ළය වරල් අනෙකුත් මෝරුන් විශේෂ අතරින් සිල්කි මෝරා හදුනා ගැනීමට උපකාරී වේ.පලමු පෘෂ්ඨීය වරල සාමාන්යයෙන් මෝරාගේ දිගෙන් දහයෙන් පංගුවක ප්රමාණයට වඩා කුඩා වේ.ඒවා පිහිටා තිබෙන්නේ ළය වරල් වල අග නිදහස් කෙළවරට පිටුපසිනි.එය කලාතුරකින් දක්නට ලැබෙන වක්රාකර එස් අකුරක (S) හැඩයක් ගනී.දෙවැනි පෘෂ්ඨීය වරල තරමක් කුඩා වේ.ළය වරල් පටු දෑකැති හැඩයකින් යුතු වේ.සාමාන්යයෙන් වැඩිහිටි මත්ස්යන්ගේ ළය වරල් දිග වේ.පසුපස වරල උල් හැඩයක් දරන අතර එය පිහිටා ඇත්තේ දෙවැනි පෘෂ්ඨීය වරලට මදක් ඔබ්බෙනි. හොදින් වැඩුණු වලිගයක් බඳු පසුපස වරල කොළයක වැනි හැඩයක් ගනී.
සමෙහි සෑම ස්ථානයක්ම දියමන්තියක හැඩැති කොරපොතු වලින් ආවරණය වී ඇත.මෝරාගේ වර්ධනය සමග මෙම කොරපොතු ප්රමාණයද වැඩි වේ.සිල්කි මෝරාගේ ශරීරය මතුපිට ලෝහමය දුඹුරු රන් පැහැයක සිට අඳුරු අලු පැහැය දක්වා වෙනස් වේ.උදරය හිම මෙන් සුදු පැහැති වේ.එය ඇලපත දක්වා විහිදී යයි.වරල් (පෘෂ්ඨීය වරල හැර )අග අඳුරු පැහැති වේ.මෙය කුඩා මෝරුන්ගේ බොහෝ විට දක්නට ලැබේ.මොවුන් මිය ගිය පසු ස්වභාවික වර්ණය අඳුරු උදාසීන අලු පැහැයක් බවට පත් වේ. සාමාන්යයෙන් මීටර් 2.5 (අඩි 8.2) පමණ වැඩෙන සිල්කි මෝරාගේ දැනට වාර්තාවී ඇති උපරිම දිග මීටර් 3.5 (අඩි 11) හා උපරිම බර කිලෝ ග්රෑම් 346 වේ. තවද ගැහැණු මත්ස්යයා පිරිමි මත්ස්යයාට වඩා විශාල වේ.
සිල්කි මෝරා යනු විවෘත මුහුදේ වෙසෙන මිලියන දහසක් තරම් වූ අති විශාල ගහණයක් සහිත මෝරුන් විශේෂ තුන අතරින් එක් විශේෂයකි.අනෙකුත් විශේෂයන් දෙක හා සසදන විට ආහාර දුර්ලභ වන ගැඹුරු මුහුදු තීරයට වඩා වෙරළ අශ්රිතව පවතින ගැඹුරු මුහුදු කලාප වලින් මෙම මත්ස්යයින් විශාල වශයෙන් සොයා ගත හැකිය.සිල්කි මෝරා ඉතා කඩිසර ලෙස තොරතුරු විපරම් කරන ආක්රමණශීලී සත්ත්වයෙකි.සාමාන්යයෙන් සෙමෙන් වැඩ කරන අයුරක් පෙන්වූවද තරගකාරී අවස්ථාවල ඉතාමත් ශක්තිමත් භාවයක් පෙන්වයි.මොහු සමහර අවස්ථාවලදී උනන්දුව ඇති කරවන යමක් දුටු විට නොසැලකිලිමත් ලෙස එය වටා ගමන් කිරීම හා හිස දෙපසට පැද්දීම සිදු කරයි.නමුත් අනතුරක් හැගී ගියහොත් එකෙණෙහිම විස්මය දනවන සුළු වේගයකින් වෙනත් තැනක් කරා යමින් එයට ප්රතිචාර දක්වයි.මෙම මෝරා බොහෝ විට පාවෙන ලී කොට කැබලි නැව් හෝ බෝට්ටු රදවා තබා ඇති ලණු ආසන්නයේ දැක ගත හැකිය.
වයසින් අඩු සිල්කි මෝරුන් අන්යෝන්ය ආරක්ෂාව සලසා ගැනීමට මනාව සංවිධානය වූ කණ්ඩායමක් ලෙස සිටීමට ප්රිය කරයි.සංක්රමණය වීම් වලදී වලදී දහස් ගණනින් මෙම මෝරුන් එකට එකතු වේ.මෙම කණ්ඩායම් සමහර විට ප්රමාණයෙන් වෙනස් වේ.මොවුන්ට එක එල්ලේ සාගර මතුපිට දක්වා පිහිනා ගොස් ක්ෂණිකව නැවත ආපසු හැරී ගැඹුරු මුහුදට පිහිනීමේ පුරුද්දක් ඇත.මෙම හැසිරීමට හේතු මෙතෙක් අනාවරණය වී නොමැත.අනතුරකට මුහුණ දුන් විට ඔවුන් ශරීරය පසුපස අර්ධය සොලවමින් වල්ගය හා ළය වරල් ගසමින් හිස ආනත කර ගැනීම සිදු කරයි.ඉන්පසු ඔවුන් අනතුර ඇති දිශාවට පෙනෙන ලෙස කුඩා වළල්ලක හැඩයට දරදඬු ආකාරයට ගැස්සෙමින් චලනය වේ.
සිල්කි මෝරා නිතරම ස්කැලොප්ඩ් හැමර්හෙඩ් මෝරු විශේෂය සමග එකට කලවම් වී හිදී.තවද සීල් මත්ස්යයන්, තල්මසුන්,ඩොල්ෆින්,දළ තල්මසුන් සමගද වාසය කිරීමට කැමැත්තක් දක්වයි.තවද සිල්කි මෝරා කුඩා පයිලට් මාළුවා සමගද වෙසීමට කැමැත්තක් දක්වයි.මක් නිසාද යත් ඔවුන් රංචු වශයෙන් මෝරා ලගින් පිහිනා යාමේදී ඔවුන්ගේ සිරුරු වලට ඉහලින් පීඩනය සහිත ජල රැළිති ඇති වේ.එයට සිල්කි මෝරා ලැදියාවක් දක්වයි.එමෙන්ම ජැක් මත්ස්යයින් ඔවුන්ගේ සමෙහි ඇති කුඩා කෑම සුන්බුන් සූරා කමින් පරපෝෂිතයකු ලෙස ජීවත් වේ.
සිල්කි මෝරා විවිධ මුහුදු මට්ටම් වල ජීවත් වන කටු සහිත මසුන් ආහාරයට ගනී.බලයා,කෙළවල්ලා,මැකරල්,සාඩින්,ගල් මාළු,මුහුදු මඩකරියා,මුහුදු අගුළුවා,ආඳා වැනි මසුන් මෙන්ම දැල්ලා,කකුළුවාද ආහාරයට ගනී.මෙවැනි මාළු රංචුවක් හමු වූ විට එම ප්රදේශයට බොහෝ මොරුන් සංඛ්යාවක් ඇදී යයි.ඉන් පසු කුඩා මාළුන් රංචුව කොටු කර ගැනීමෙන් අනතුරුව එම මාළුන් රෑනම විනාශ කර දමයි.මෙලෙස කුඩා ප්රදේශයකට කොටු වන මාළු රෑනට විවෘත කරගත් මුඛයෙන් යුතුව දිගින් දිගටම පහර දීම සිදු කරයි.මෙම ප්රහාරයට ලක් වන කුඩා මසුන් ඔවුන්ගේ හකු කොනෙන් අල්ලා ගනී. බොහෝ පිරිසක් කුඩා මාළු රංචුවකින් ආහාර සපයා ගත්තද පහර දීම තනි තනිව සිදු කරයි. බහමාස් හා වෙරළබඩ ෆ්ලොරීඩාවේ කරන ලද අධ්යයන වලට අනුව පෙනී ගොස් ඇත්තේ සිල්කි මෝරා ශබ්දයට ඉතාම සංවේදී වන බවයි.එනම් අඩු සංඛ්යාත(10 - 20 හර්ට්ස්) හා අසාමාන්ය ස්ඵන්ද වලට ඔවුන් ස්ංවේදී වේ.පර්යේෂණ වලදී ගැඹුරු ජලයේදී නිකුත් කල ශබ්ද වලට මීටර් සිය ගණනක් එපිට මෝරුන්ද ආකර්ෂණය කර ගැනීමට හැකිවිය.එම ශබ්දය ඔවුන්ගේ ආහාර ලෙස ගන්නා සතුන්ගේ සහ කුරුල්ලන්ගේ ඩොල්ෆින් මසුන්ගේ ශබ්දයට සමාන ශබ්ද විය.එහිදී ඔවුන් ඊට තම ආහාර ලෙස රැවටී එයට ඒකරාශී විය. මෙම අධ්යයන වලදී තවද හෙලි වූයේ උස් පහත් හඩවල් හෝ වෙනත් ශබ්ද නිකුත් කලද මෝරාගේ ප්රතිචාරයේ වෙනසක් සිදු නොවු බවයි.නැවතත් කල පරීක්ෂණ වලින් හෙලිවූයේද ශබ්ද වල වෙනසක් ඇතිවීමෙන් මෝරා පසුබැසීමකට ලක් නොවන බවයි.
සිල්කි මෝරා ආසන්න වශයෙන් නිව්ටන් 890ක් ලෙස ගණන් බලා ඇත.මොවුන් බලයන් හා කෙළවල්ලන් සමග සමීපව වාසය කරයි.මෙම මසුන් රංචු 'ටූනා' රංචු ලෙස හදුන්වයි.ඝානාවේදී හමු වන බොහෝ ටූනා රංචු පිටුපසින් සිල්කි මෝරුන් කිහිප දෙනෙක් ගැවසෙනු දැක ගත හැකිය. නැගෙනහිර පැසිෆික් සාගරයේ ටූනා මසුන් මැරීමට යන ධීවරයින්ගේ දැල් ආදී ධීවර ආම්පන්න වලට මොවුන් හානි කරන බවද පැවසේ.එබැවින් එම ධීවරයන් 'දැල් කන මෝරා ' ලෙස මෙම මෝරා අමතයි. සිල්කි මෝරා, බෝතල් හැඩැති නාසයක් සහිත ඩොල්ෆින් මසුන් සමග තරග කරන අවස්ථාවන් දක්නට ලැබේ.එලෙස තරග වදිනුයේ දෙපිරිසම එකම මාළු විශේෂයක් ගොදුරු කර ගැනීමට උත්සහ දරණ විටදීය.මෙහිදී ඩොල්ෆින් මාළුන්ට ලැබෙන ආහාර ප්රමාණය රදා පවතිනුයේ අනෙක් පස සිටින සිල්කි මෝරුන්ගේ ගණන මතයි.මෙහි විශාල මෝරුන් ප්රමාණයක් සිටී නම් ඔවුන් මාළු රංචුව මධ්යස්ථයේ සිටිමින් ඩොල්ෆින්ට පිටත සීමාව ලබා දේ.එබැවින් මෝරුන්ගේ දරුණු පහරදීම් වලින් සිදු වන අනතුරු වැළකීම සිදුවේ.නමුත් ඩොල්ෆින් මසුන් ප්රමාණය වැඩි නම් ඔවුන් සිල්කි මෝරුන් කුඩා මාළු රංචුව වෙතින් පළවා හැරීම සිදු කරයි.මින් කුමන වර්ගයක් වැඩි ප්රමාණයකින් සිටියද මෙම ගොදුරු සොයන්නන් දෙවර්ගය ප්රසිද්ධියේ එකිනෙකා සමඟ කලහ ඇති කර නොගනී.
සිල්කි මෝරාද අනෙකුත් මෝර විශේෂ මෙන්ම ජලාබුජ සත්වයෙකි.කළලය මවගේ වැදෑමහ ඔස්සේ පෝෂණය ලබයි.සිල්කි මෝරාගේ කළල බන්ධනය ක්ෂීරපායී ආකෘතියට ආසන්න සමාන බවක් දක්වයි.එනම් අනෙකුත් ක්ෂීරපයී සත්වයන්ගේ මෙන් මව හා භ්රෑණය අතර පවතින ඉතා සියුම් පටලය මෙම සත්ත්වයාට පිහිටා නොමැත.තව දුරටත් සොයා බැලීමේදී කළලය සතු වන රතු රුධිරාණු සෛල මවු පාර්ශවීය රතු රුධිරාණු සෛල වලට වඩා කුඩා වේ.මෙම ලක්ෂණය සාමාන්ය ක්ෂීරපායී සත්ත්වයන්ට වඩා වෙනස් වූවකි.වැඩුණු ගැහැණු සතුන් සතුව එක් ඩිම්බකෝශයක් (දකුණු පස ) හා ගර්භාශ දෙකක් පවති. ඒවා එකිනෙක භ්රෑණයන් සදහා වෙන් වෙන්ව බෙදී පවතී.
සිල්කි මෝරාගේ ප්රජනනය සිදු වනුයේ බොහෝ විට වසරකට වතාවකි. ගල්ෆ් මෙක්සිකෝවේ ජීවත් වන සිල්කි මෝරුන්ගේ සංසර්ගය හා පැටව් බිහි වීම සිදු වනුයේ වසන්තයේ අග භාගයේ වේ.ග්රීෂ්ම ඍතුවේ මුල් කාලයේදීය. (මැයි සිට අගෝස්තු ) ගැහැණු සත්ත්වයා පැටවුන් බිහි කරනුයේ මාස 12ක් ගර්භණී කාලයකට පසුවය.එය සෑම වසරක් පාසා හෝ වසරක් හැර වසරක සිදු කරයි.පැටවුන් සංඛ්යාව 1-16 දක්වා ප්රමාණයක් එක වර බිහි කරයි.එය ගැහැණු සත්ත්වයගේ විශාලත්වය මත 6 සිට 12 දක්වා වීම සාමාන්යයෙන් සිදුවේ. පැටවුන් බිහි කිරීම සිදු වනුයේ මහද්වීපික තටකයට ඔබ්බෙන් වූ ගල්පර ආශ්රිතවය.අළුත ඉපදුණු පැටවුන්ට වැඩුණු මෝරුන් විසින් ආහාර හා ආරක්ෂාව සැපයීම සිදු කරයි. ඉපදී පළමු වසර තුල මොවුන් සෙන්ටි මීටර් 25-30ක් (අඟල් 9.8-11.8)පමණ වර්ධනය වේ.මෙම පැටවුන් මාස කිහිපයකට පසු ගල්පර සහිත නොගැඹුරු මුහුදු තීරයෙන් ගැඹුරු මුහුදට සංක්රමණය වේ.
වයඹ දිග අත්ලාන්තික් සාගරයේ මෝරුන් අනෙකුත් මෝරුට වඩා වර්ධනය වීමේ නැඹුරුතාවක් දක්වයි.අනෙකුත් මෝරු විශේෂයන්ට සාපේක්ෂව ගැහැණු හා පිරිමි දෙපිරිසම සමාන වර්ධන අනුපාතක් හිමි කර ගනී. මධ්යම පැසිෆික් සාගරයේ ගැහැණු සතුන්ගේ වර්ධන වේගය පිරිමි සතුන්ගේ වර්ධන වේගයට වඩා අඩු වේ.තවද ශීත කලාපීය ජලයෙහි ජීවත් වන මෝරුන් උණුසුම් කලාපයේ ජීවත් වන මොරුන්ට වඩා සෙමෙන් වර්ධනය වේ.පිරිමි සතුන් අවුරුදු 6-10 සීමාවේදී හා ගැහැණු සතුන් 7-12 සීමාවේදි ලිංගික පරිණතභාවයට පත්වේ.මොවුන්ගේ උපරිම ආයු කාලය අවුරුදු 22ක් පමණ වේ.
සිල්කි මෝරාගේ සාමාන්යය ජීවන තොරතුරු
සිල්කි මෝරාගේ සිරුරේ විශාලත්වය හා දත් වල පිහිටීම නිසා මනුෂ්යයාට අවදානම් සත්ත්වයකු ලෙස හදුන්වයි.කෙසේ වුවද ඔවුන් මිනිසුන් හමුවට එනුයේ මිනිසුන් ඔවුන්ගේ වාසස්ථාන වලට ඇතුළු වූ අවස්ථාවන්හිදීය.ආහාරයක් දැකීමෙන් ඔවුන් භයානක ලෙස කලබල වීම සිදුවේ.ස්වාභාවික කුතුහලයකින් හා නිර්භීතකමකින් ඔවුන් කිමිදුම් කරුවන් වෙත ලගා වේ.විවෘත ගැඹුරු මුහුදට වඩා ගල් පර අශ්රිතව මොවුන්ට කිමිදුම්කරුවන් හමු වූ විට වඩාත් කළහකාරී ලෙස හැසිරේ.සමහර අවස්ථාවලදී තනිව සිටින සිල්කි මෝරෙකුට කිමිදුම්කරුවෙක් හමු වූ විට මුරණ්ඩු ලෙස කරදර කොට ඔවුන් ජලයෙන් පන්නා දැමීමට තරම් දරුණු වේ.ජාත්යන්තර මෝරුන් පහර දීමේ තොරතුරු ලේඛනයේ ( International Shark Attack File ) සිල්කි මෝරා විසින් සිදු කරනු ලැබූ පහර දීම් ගණන 6කි.ඉන් 3ක් බරපතල ගණයේ පහරදීම් නොවේ.
මෙක්සිකෝ, ග්වාතමාලා, එල් සැල්වදොර්, කොස්ටරීකා,ඇමෙරිකා එක්සත් ජනපදය ,ඉක්වදෝර්, ස්පාඤ්ඤය, පෘතුගාලය, ශ්රී ලංකාව,මාලදිවයින, යේමනය වැනි රටවල් වල සිදු කරන වාණිජමය මත්ස්ය කර්මාන්තයේදී විශාල වශයෙන් සිල්කි මෝරා අල්ලා ගනු ලැබේ.තවද ටූනා මසුන් ඇල්ලීමට යොදා ගන්නා දැල් වලට අහඹු ලෙස හසු වීමෙන් සැලකිය යුතු සිල්කි මෝරුන් ප්රමාණයක් මිය යයි.නැගෙනහිර පැසිෆික් කලාපයේ බහුලවම සිදු වන ටුනා මසුන් කර්මාන්තයේදී මෙලෙස අහඹු ලෙස හසු වීමෙන් මිය යන මෝරුන් අතර නිල් මෝරාට පළමු තැන හිමිවේ.දෙවැනි තැන ලැබෙනුයේ සිල්කි මෝරාටය.මොවුන්ගේ වරල් මෝර වරල් සුප් වලට යොදා ගනී.ඒ සදහා මිනිසුන් මෙම මසුන් මුහුදේදීම මරා වරල් කපා ඉතිරි සිරුරු කොටස් ඉවත දැමීම සිදු කරයි. නමුත් මේ නිසා ලෝකය පුරා සිල්කි මෝරුන් මිලියන 1.5ක් පමණ වාර්ෂිකව මිය යන බව ගණන් බලා ඇත.මොවුන්ගේ මස්(අලුත් හෝ ලුණුයොදා වියළනලද ),සම,මෝර තෙල්ද හකුද ප්රයෝජනයට ගනු ලැබේ.මෙම මෝරුන්ගේ හකු විකිණීම සංචාරක කර්මන්තයේදී ප්රධාන තැනක් ගනී.සමහර අවස්ථා වල ක්රීඩාවක් ලෙස මසුන් ඇල්ලීම කරන පිරිස් නිසාවෙන්ද සිල්කි මෝරුන්මිය යාම සිදුවේ.
ලෝකය පුරා ඉතා බහුල ලෙස පැතිරී සිටින සිල්කි මෝරා ඉතා හොද ප්රතිශක්තියකින් යුතු මත්ස්යයෙකි.නමුත් මහා පරිමාණ මත්ස්යය කර්මාන්තය නිසා මොවුන් අධික ලෙස අඩු වෙමින් පවතී.දකුණු හා මධ්යම පැසිෆික් සාගර වල ටූනා කර්මාන්තයේදී 1989දී පමණක් මියගිය සිල්කි මෝරුන්ගේ සංඛ්යාව 900,000 ක් පමණ වේ.නමුත් එමගින් ඔවුන්ගේ ගහණයට සැළකිය යුතු බලපෑමක් එල්ල නොවිනි.මෙවැනි අවස්ථා පිළිබද ලැබෙන දත්ත ප්රමාණවත් නොවීම හා මෝරුන් වර්ග එකිනෙක වෙන්කොට හදුනා ගැනීමට ධීවරයන්ට අත්දැකීම් මදි කම නිසා නිවැරදි සංඛ්යා ලේඛන ලබා ගැනීමට නොහැකි තත්වයක් උදා වී ඇත.කෙසේ වුවද අඩු වී යන සිල්කි මෝරුන් ප්රමාණය නැවත ප්රජනනය මගින් සමතුලිත කිරීමට නොහැකි වී ඇත.වාර්ශිකව සිල්කි මෝරුන් මිය යන ප්රමාණය ආහාර හා කෘෂිකර්මාන්ත සංවිධානයට ( Food and Agricultural Organization (FAO) ) වාර්තා වූ අකාරයට 2000 වසරේදී ටොන් 11,680ක් පමණ වූ පරිභෝජනය 2004 වසරේදී ටොන් 4358 දක්වා පහල ගොස් තිබිණි.මෝරුන් පරිභෝජනය පිළිබද ප්රාදේශීය මට්ටමින් සිදු වූ ගණනය කිරීම් අනුව 1950 සිට 1990 දක්වා මධ්යම පැසිෆික් වලින් 90%ක්ද ,1991 සිට 2000 දක්වා කොස්ටරීකා වලින් 60%ක්ද 1950 සිට 1990 දක්වා ගල්ෆ් මෙක්සිකෝවේ 91%ක්ද (සියළු මෝරුන් විශේෂ ) 1986 සිට 2005 දක්වා වයඹ දිග අත්ලාන්තික් වලින් 85%ක්ද අඩු වී ගොස් ඇත.ශ්රි ලංකාවෙන් වාර්තා වූ අන්දමට සිල්කි මෝර මත්ස්යය කර්මාන්තයේ උපරිම අල්ලා ගැනීම 1996දී ටොන් 25400 සිට 2006දී ටොන් 1960 දක්වා අවම වී ඇත.ඊට හේතුව දේශීය වෙළද පොල ඉල්ලුම අඩු වීමයි. නමුත් 1970 සිට 1990 දක්වා ජපනයේ හා ඉන්දීය හා පැසිෆික් මුහුදු වල සිදුවන සිල්කි මෝර කර්මන්තයේ පහල බැසීමක් දක්නට නොලැබේ.එමෙන්ම ගල්ෆ් මෙක්සිකෝවේ සහ උතුරු අත්ලාන්තික් සාගරයේ මෙම අගයන් ගණනය කිරීමට යොද ගත් ක්රියාවලි නිවැරදිද යන්න විවාදයට තුඩු දෙන කරුණක් වී ඇත.
දළ වශයෙන් සොයා ගත් තොරතුරු අනුව 2007දී International Union for Conservation of Nature (IUCN) මගින් සිල්කි මෝරා වඳ වීමට ආසන්න තත්ත්වයකට පත් මත්ස්යයෙක් ලෙස නම් කර ඇත. සිල්කි මෝරා ප්රාදේශිය මට්ටමින් නිරිත දිග අත්ලාන්තික් සාගරය,ඉන්දියන් සාගරය, නැගෙනහිර, මධ්යම,දකුණු පැසිෆික් සාගරය වැනි කලාප ආශ්රිතව තර්ජනයට ලක් වී ඇති බව සොයා ගෙන ඇත. මෙම මසුන්ගේ වරල් කැපීම තහනම් කර ඇති අතර එසේ කරන්නේ නම් එය ජාතික හෝ ජාතික සීමාව ඉක්මවා යන වරදක් බව United Nations Convention on the Law of the Sea ප්රකාශයට පත් කර ඇත.International Commission for the Conservation of Atlantic Tunas (ICCAT) ,Inter-American Tropical Tuna Commission (IATTC) වැනි සංවිධාන ටුනා කර්මාන්තයේදී සිල්කි මෝරුන් අහඹු ලෙස හසු වීමෙන් මිය යාම වැළැක්වීමට ක්රියාමාර්ග ගෙන ඇත.ටුනා මසුන් සමඟ සිල්කි මෝරා සංක්රමණය වීම සුලභව සිදු වේ.එනිසා වාණිජමය මත්ස්ය කර්මාන්තය හමුවේ අහඹු ලෙස සිල්කි මෝරා මිය යාම අවම කර ගැනීමට සරල ක්රියාමාර්ගයක් ගැනීමට නොහැකි වී ඇත.
{{reflist|colwidth=30em|refs= [1]
සිල්කි මෝරා යනු කාකරයිනිඩයි පවුලට (Carcharhinus falciformis) අයත් ක්ෂීරපායී මෝරුන් විශේෂයකි.සමෙහි ස්වභාවය අනුව ඔවුන් මේ ලෙස නම් කර ඇත.නිවර්තන කලාපීය සාගර වල නොගැඹුරු මුහුදේ මෙම මෝරුන් විශේෂය සුලබ වශයෙන් දක්නට ඇත.ඉතා චංචල ස්වභාවයක් ඇති මෙම මෝරා මහද්වීපික තටකයට මීටර් 50ක් (අඩි 164ක්) පමණ ගැඹුරු මුහුදේ දී දැක ගත හැකිය. අනාකූල සිහින් සිරුරකට හිමිකම් කියන මෙම මත්ස්යයා සාමාන්යයෙන් මීටර් 2.5ක් (අඩි 8යි අඟල් 2යි ) පමණ වර්ධනය වේ.සිරුර පසුපස ඇති වක්රාකාර දාරයක් සහිත කුඩා පෘෂ්ඨීය වරල නිසා මොවුන් අනෙක්කුත් මෝරන් අතරින් විශේෂ කොට හදුනා ගත හැක. එමෙන්ම නිදහස් ලෙස විහිදී ගිය දෑකැත්තක හැඩැති ලය වරල් යුගලයක්ද මෙම මත්සයාට ඇත.ඒවා මතුපිට ලෝකඩමය අළු පැහැයක් ඇති අතර යට ප්රදේශය සුදු පැහැයක් ගනී.
මොවුන් වෙසෙන සාගරික කලාපයේ ගොදුරු හිඟ වූ විට මොවුන් ඉතා වේගවත් මුරණ්ඩු ස්වභාවයකින් යුතු දඩයක්කාරයකු ලෙස හැසිරේ.මෙම මෝරු විශේෂය ප්රධාන වශයෙන් කටු සහිත මසුන් සහ සෙෆලපොඩ් නැමැති මසුන් විශේෂය ආහාරය ලෙස ලබා ගනී.තම මුඛය පුළුල් ලෙස විවෘත කරමින් කුඩා මසුන් රෑනක් එකවර හඹා ගොස් දරුණු ප්රහාරයක් එල්ල කිරිම සිදු කරයි.එමෙන්ම මොවුන් බලයා,කෙළවල්ලා වැනි මසුන්ගේ ඉව ඔස්සේ හඹා ගොස් ගොදුරු කර ගැනීමටද කැමත්තක් දක්වයි.ශ්රවණ සන්වේදනය ඉතාම තියුණු වන මෙම සත්වයාට තමන් ගොදුරු කර ගන්නා කුඩා වර්ගයේ මාළුන් විසින් ඇති කරන අඩු සංඛ්යාත හඩවල් ඔස්සේ යමින් ආහාර ප්රභව සොයා ගැනීම ඉතා පහසුවෙන් සිදු කරයි.
සිල්කි මෝරා ජලාබුජ සත්වයෙකි.එනම් කළලය පෝෂණය වන්නේ කළල බන්ධනය මගින් මවට සම්බන්ධ වීමෙනි.මෙය ඔවුන්ගේ ජීවිත කාලයේ එක් විශේෂ අවස්ථාවකි.මෙම සතුන්ගේ ප්රජනන ක්රියාවලිය ඍතු චක්රය අනුව සිදු වේ. මෙක්සිකෝවේ ගල්ෆ් කලාපයේ වෙසෙන ගැහැණු සතුන් හැර අනෙකුත් සාගර කලාප වල ජීවත් වන ගැහැණු සත්ත්වයා ප්රජනනයේදී වාර්ෂිකව හෝ ද්විවාර්ෂිකව පැටවුන් 16 දක්වා පමණ බිහි කරයි.අලුතින් බිහි වන පැටවුන් ගැඹුරු මුහුදෙහි ජීවත් වීමට තරම් වැඩෙන තෙක් මුල් මාස කිහිපය ගොඩබිමට ඈතින් ඇති ගල්පර ආශ්රිතව ගැවසෙමින් ජීවත් වේ.
සමහර අවස්ථා වලදී කිමිදුම් කරුවන්ටද ආක්රමණශීලී ප්රහාර එල්ල කරන මෙම මත්සයා සතු විශාල කැපෙන සුළු දත් නිසා ඔවුන්ට තවත් භයානක පෙනුමක් ලැබී ඇත.කෙසේ වුවද මිනිසුන් ඔවුන්ගේ වාසස්ථාන වලට ඇතුල් වූ අවස්ථා කිහිපයකදී හැර අනෙකුත් අවස්ථා වල එල්ල කරන ලද ප්රහාර දුර්ලභ වේ. සිල්කි මෝරාගේ වරල් ඉතා වටිනාකමින් වැඩි අතර සම,මෝර තෙල්,හකු හා මස්ද ලබා ගනී.මොවුන් ඉතා සුලභ බැවින් බොහෝ රටවල් වල මත්ස්ය කර්මාන්තයේ ප්රධාන සංරචකයක් ලෙස වැඩි වාණිජ වටිනාකමක් හිමි කර ගනී.තවද සිල්කි මෝරා බලයා,කෙලවළ්ලා වැනි මාළු විශේෂ සමඟ නිරන්තරයෙන් ගැවසෙන නිසා බලයන් කෙලවළ්ලන් ඇල්ලීම සිදු කරන බොහෝ අවස්ථා වල අහඹු ලෙස මෝරුන්ද දැල් වලට හසු වීම සිදු වේ.අනෙකුත් මෝරුන්ගේ මෙන්ම ප්රජනන ක්රියාවලිය සෙමෙන් සිදු වුවද සිල්කි මෝරා බොහෝ ප්රදේශයක පැතිරී ගිය සාමාන්ය ගහණයකින් යුතු මෝරුන් විශේෂයකි.නමුත් පසුගිය කාල වකවානුව තුල මත්ස්ය කර්මාන්තය හේතුවෙන් ඔවුන්ගේ ගහණය අඩු වී යන බවට ගණන් බලා ඇත. 2007 වසරේදී International Union for Conservation of Nature (IUCN) මගින් නිකුත් කර ඇති සංඛ්යා ලේඛන අනුව මෙම මෝරුන්ගේ සංරක්ෂණ තත්වය අවම වැදගත් සිට තර්ජනයට ලක්වීමට ආසන්න ලෙස ප්රකාශයට පත් කර ඇත.
The silky shark (Carcharhinus falciformis), also known by numerous names such as blackspot shark, gray whaler shark, olive shark, ridgeback shark, sickle shark, sickle-shaped shark and sickle silk shark, is a species of requiem shark, in the family Carcharhinidae, named for the smooth texture of its skin. It is one of the most abundant sharks in the pelagic zone, and can be found around the world in tropical waters. Highly mobile and migratory, this shark is most often found over the edge of the continental shelf down to 50 m (164 ft). The silky shark has a slender, streamlined body and typically grows to a length of 2.5 m (8 ft 2 in). It can be distinguished from other large requiem sharks by its relatively small first dorsal fin with a curving rear margin, its tiny second dorsal fin with a long free rear tip, and its long, sickle-shaped pectoral fins. It is a deep, metallic bronze-gray above and white below.
With prey often scarce in its oceanic environment, the silky shark is a swift, inquisitive, and persistent hunter. It feeds mainly on bony fishes and cephalopods, and has been known to drive them into compacted schools before launching open-mouthed, slashing attacks. This species often trails schools of tuna, a favored prey. Its sense of hearing is extremely acute, allowing it to localize the low-frequency noises generated by other feeding animals, and, by extension, sources of food. The silky shark is viviparous, meaning that the developing embryos are sustained by a placental connection to their mother. Significant geographical variation is seen in its life history details. Reproduction occurs year-round except in the Gulf of Mexico, where it follows a seasonal cycle. Females give birth to litters of up to 16 pups annually or biennially. The newborn sharks spend their first months in relatively sheltered reef nurseries on the outer continental shelf, growing substantially before moving into the open ocean.
The large size and cutting teeth of the silky shark make it potentially dangerous, and it has behaved aggressively towards divers. However, attacks are rare, as few humans enter its oceanic habitat. Silky sharks are valued for their fins, and to a lesser extent their meat, hide, liver oil, and jaws. Because of their abundance, they form a major component of commercial and artisanal shark fisheries in many countries. Furthermore, their association with tuna results in many sharks being taken as bycatch in tuna fisheries. Although slow-reproducing like most other sharks, the wide distribution and large population size of the silky shark was once thought to buffer the species against these fishing pressures. However, data now suggest that silky shark numbers are declining around the world, which prompted the IUCN to reassess its conservation status to Vulnerable in 2017.
A scientific description of the silky shark was first published by the German biologists Johannes Müller and Jakob Henle under the name Carcharias (Prionodon) falciformis, in their 1839 Systematische Beschreibung der Plagiostomen. Subsequent authors have assigned this species to the genus Carcharhinus.[5][6] Because Müller and Henle's type specimen was a 53-cm-long female fetus from Cuba, adult silky sharks were historically not recognized as C. falciformis and were described as a separate species, Carcharhinus floridanus, by Henry Bigelow, William Schroeder, and Stewart Springer in 1943. Jack Garrick, Richard Backus, and Robert Gibbs Jr. synonymized C. floridanus with C. falciformis in 1964.[7]
The specific epithet falciformis is Latin for "sickle-shaped", which refers to the outline of the dorsal and pectoral fins.[3] The silky shark's common name comes from the fine texture of its skin compared to other sharks, a product of its tiny, densely packed dermal denticles.[8] It may also be referred to as blackspot shark (usually used for C. sealei), grey reef shark (usually used for C. amblyrhynchos), grey whaler shark, olive shark, reef shark, ridgeback shark, sickle shark, sickle silk shark, sickle-shaped shark, silk shark, and silky whaler.[9]
Carcharhinus falciformis
Phylogenetic relationships of the silky shark, based on allozyme sequences[10]Fossilized teeth belonging to the silky shark have been found in North Carolina: from the vicinity of two baleen whales, one in mud dating to the Pleistocene-Holocene (circa 12,000 years ago) and the other in Goose Creek Limestone dating to the Late Pliocene (circa 3.5 million years ago – Mya), as well as from the Pungo River, dating to the Miocene (23–5.3 Mya).[11][12] Fossil teeth have also been found in Pliocene strata at the Cava Serredi quarry in Tuscany, Italy.[13] Carcharhinus elongatus, an earlier representative of its lineage with smooth-edged teeth, is known from Oligocene (34–23 Mya) deposits in the Old Church formation of Virginia, and the Ashley formation of South Carolina. A set of poorly described, Eocene (56–34 Mya) teeth resembling those of this species are known from Egypt.[12]
Initial efforts to resolve the evolutionary relationships of the silky shark were inconclusive; based on morphology, Jack Garrick in 1982 suggested the blackspot shark (C. sealei) as its closest relative.[14] In 1988, Leonard Compagno assigned it phenetically to an informal "transitional group" also containing the blacknose shark (C. acronotus), the blacktip reef shark (C. melanopterus), the nervous shark (C. cautus), the copper shark (C. brachyurus), and the night shark (C. signatus).[15]
More recently, Gavin Naylor's 1992 phylogenetic analysis, based on allozyme sequence data, found that the silky shark is part of a group containing large sharks with a ridge between the dorsal fins. One branch within this group contains the sandbar shark (C. plumbeus) and the bignose shark (C. altimus), while the silky shark is the basal member of the other branch and the sister taxon to a clade containing the Caribbean reef shark (C. perezi), Galapagos shark (C. galapagensis), oceanic whitetip shark (C. longimanus), dusky shark (C. obscurus), and blue shark (Prionace glauca).[10] Mine Dosay-Abkulut's 2008 ribosomal DNA analysis, which included the silky, blue, and bignose sharks, confirmed the closeness of those three species.[16]
The silky shark has a cosmopolitan distribution in marine waters warmer than 23 °C (73 °F). In the Atlantic Ocean, it is found from Massachusetts (USA) to Spain in the north, and from southern Brazil to northern Angola in the south, including the Gulf of Mexico and the Caribbean Sea. In the Mediterranean Sea, it has been recorded first in the Alboran Sea, subsequently in Algerian waters, the Gulf of Gabes (Tunisia) and more recenly in the Ligurian Sea.[17] It occurs throughout the Indian Ocean, as far south as Mozambique in the west and Western Australia in the east, including the Red Sea and the Persian Gulf. In the Pacific Ocean, the northern extent of its range runs from southern China and Japan to southern Baja California and the Gulf of California, while the southern extent runs from Sydney, Australia, to northern New Zealand to northern Chile.[3][5] Based on life history differences, four distinct populations of silky sharks have been identified in ocean basins worldwide: in the northwestern Atlantic, in the western and central Pacific, in the eastern Pacific, and in the Indian Ocean.[3]
Primarily an inhabitant of the open ocean, the silky shark is most common from the surface to a depth of 200 m (660 ft), but may dive to 500 m (1,600 ft) or more.[5] Tracking studies in the tropical eastern Pacific and northern Gulf of Mexico have found that cruising silky sharks spend 99% of their time within 50 m (160 ft) of the surface, and 80–85% of their time in water with a temperature of 26–30 °C (79–86 °F); the pattern was constant regardless of day or night.[18][19] This species favors the edges of continental and insular shelves, often over deepwater reefs and around islands. Its range extends farther north and south along continental margins than in oceanic waters. On occasion, it may venture into coastal waters as shallow as 18 m (59 ft).[20] Silky sharks are highly mobile and migratory, though the details of their movements are little-known. Tagging data have recorded individual sharks moving up to 60 km (37 mi) per day, and covering distances up to 1,339 km (832 mi).[21] Larger sharks generally move longer distances than smaller ones. In the Pacific Ocean and possibly elsewhere, it spends the summer at slightly higher latitudes, particularly during warmer El Niño years.[22][23] In the northern Atlantic, most sharks follow the Gulf Stream northward along the U.S. East Coast.[21] In the Gulf of Aden, it is most common in late spring and summer.[3]
Slim and streamlined, the silky shark has a fairly long, rounded snout with barely developed flaps of skin in front of the nostrils. The circular, medium-sized eyes are equipped with nictitating membranes (protective third eyelids). Short, shallow furrows are present at the corners of the mouth.[5][24] 14-16 and 13–17 tooth rows are found on either side of the upper and lower jaws, respectively (typically 15 for both). The upper teeth are triangular and strongly serrated, with a notch in the posterior edge; they are erect at the center and become more oblique towards the sides. The lower teeth are narrow, erect, and smooth-edged. The five pairs of gill slits are moderate in length.[25]
The dorsal and pectoral fins are distinctive and help to distinguish the silky shark from similar species. The first dorsal fin is relatively small, measuring less than a tenth as high as the shark is long, and originates behind the free rear tips of the pectoral fins. It has a rounded apex, an "S"-shaped rear margin, and a free rear tip about half as long as the fin is tall. The second dorsal fin is tiny, smaller than the anal fin, with a drawn-out free rear tip up to three times as long as the fin is tall. A narrow dorsal ridge runs between the dorsal fins. The pectoral fins are narrow and sickle-shaped, and particularly long in adults. The anal fin originates slightly ahead of the second dorsal fin and has a deep notch in the posterior margin. The caudal fin is fairly high with a well-developed lower lobe.[5][24]
The skin is densely covered by minute, overlapping dermal denticles. Each dermal denticle is diamond-shaped and bears horizontal ridges leading to posterior marginal teeth, which increase in number as the shark grows.[7][8] The back is metallic golden-brown to dark gray and the belly is snowy white, which extends onto the flank as a faint lighter stripe. The fins (except for the first dorsal) darken at the tips; this is more obvious in young sharks.[5][8] The coloration quickly fades to a dull gray after death.[26] One of the largest members of its genus, the silky shark commonly reaches a length of 2.5 m (8.2 ft), with a maximum recorded length and weight of 3.5 m (11 ft) and 346 kg (763 lb), respectively.[9] Females grow larger than males.[8]
The silky shark is one of the three most common pelagic sharks along with the blue and oceanic whitetip sharks, and counts among the most numerous large oceanic animals in the world with a population of at least tens of millions.[27] Compared to the other two species, it is less strictly pelagic with the greatest numbers found in offshore waters associated with land, where food is more readily obtained than farther out in the truly open ocean. The silky shark is an active, inquisitive, and aggressive predator, though it will defer to the slower but more powerful oceanic whitetip shark in competitive situations.[5] When approaching something of interest, it may seem inattentive, sedately circling and sometimes swinging its head from side to side. However, it can respond with startling swiftness to any shift in its immediate surroundings.[28] This shark is often found around floating objects such as logs or tethered naval buoys.[29]
Younger silky sharks are known to form large, loosely organized aggregations, possibly for mutual defense.[30] During migrations, over a thousand individuals may gather.[31] These groups are generally segregated by size, and in the Pacific perhaps also by sex.[8][22][32] Silky sharks within a group have been observed to "tilt", presenting their full lateral profile towards each other, as well as gape their jaws or puff out their gills. On occasion, sharks have also been seen suddenly charging straight up, veering away just before reaching the surface and gliding back down to deeper water. The significance of these behaviors is unknown.[28] When confronted, the silky shark may perform a threat display, in which it arches its back, drops its tail and pectoral fins, and elevates its head. The shark then proceeds to swim in tight loops with a stiff, jerky motion, often turning broadside towards the perceived threat.[33]
Potential predators of the silky shark include larger sharks and killer whales (Orcinus orca).[34] Known parasites of this shark include the isopod Gnathia trimaculata,[35] the copepod Kroeyerina cortezensis,[36] and the tapeworms Dasyrhynchus variouncinatus and Phyllobothrium sp.[37][38] Silky sharks frequently intermingle with schools of scalloped hammerheads (Sphyrna lewini), and have been known to follow marine mammals. One account from the Red Sea describes 25 silky sharks following a large pod of bottlenose dolphins (Tursiops sp.), along with 25 grey reef sharks (C. amblyrhynchos) and a lone silvertip shark (C. albimarginatus). Silky sharks are themselves accompanied by juvenile pilot fish (Naucrates ductor), which "ride" the pressure wave ahead of the shark, as well as by jacks, which snatch scraps of food and rub against the shark's skin to scrape off parasites.[30][39]
The silky shark is an opportunistic predator, feeding mainly on bony fishes from all levels of the water column, including tuna, mackerel, sardines, mullets, groupers, snappers, mackerel scads, sea chubs, sea catfish, eels, lanternfishes, filefishes, triggerfishes, and porcupinefishes. It may also take squid, paper nautilus, and swimming crabs, and fossil evidence indicates it scavenged on whale carcasses.[3][5][11] Good feeding opportunities can draw silky sharks in large numbers; one such feeding aggregation in the Pacific has been documented "herding" a school of small fishes into a compact mass (a bait ball) and trapping it against the surface, whereupon the sharks consumed the entire school.[3] When attacking tightly packed fish, silky sharks charge through the ball and slash open-mouthed, catching the prey fish at the corners of their jaws. Although multiple individuals may feed at once, each launches its attack independently.[30]
Studies conducted off the Florida coast and the Bahamas have shown that silky sharks are highly sensitive to sound, in particular low-frequency (10–20 Hz), irregular pulses. Experiments in which these sounds were played underwater attracted sharks from hundreds of meters away. Silky sharks likely orient to these sounds because they are similar to the noise generated by feeding animals such as birds or dolphins, thus indicating promising sources of food.[28][30] These studies have also demonstrated that a silky shark attracted by one sound will quickly withdraw if that sound abruptly changes in amplitude or character; this change need not be a sound produced by a predator to evoke the reaction. Over repeated exposures, silky sharks habituate to the sound change and stop withdrawing, though it takes them much longer to do so compared to the bolder oceanic whitetip shark.[34]
The bite force of a 2-m-long silky shark has been measured at 890 newtons (200 lbf).[40] A well-established association exists between this species and tuna: off Ghana, almost every tuna school has silky sharks trailing behind, and in the eastern Pacific, these sharks inflict such damage to tuna fishing gear and catches that fishery workers have given them the moniker "net-eating sharks".[5][26] Silky sharks and bottlenose dolphins compete when both species target the same school of fish; the amount eaten by the dolphins decreases relative to the number of sharks present. If a large number of sharks is present, they tend to remain inside the prey school, while the dolphins consign themselves to the periphery, possibly to avoid incidental injury from the sharks' slashing attacks. Conversely, if a large enough group of dolphins gathers, they become able to chase the sharks away from the prey school. Regardless of which one dominates, the two predators do not engage in any overtly aggressive behavior against each other.[41]
Like other members of its family, the silky shark is viviparous: once the developing embryo exhausts its supply of yolk, the depleted yolk sac is converted into a placental connection through which the mother delivers nourishment. Relative to other viviparous sharks, the placenta of the silky shark is less similar to the analogous mammalian structure in that no interdigitation exists between the tissues of the fetus and mother. Furthermore, the fetal red blood cells are much smaller than maternal blood cells, which is opposite the pattern seen in mammals. Adult females have a single functional ovary (on the right side) and two functional uteri, which are divided lengthwise into separate compartments for each embryo.[42]
Silky sharks in most parts of the world are thought to reproduce year-round, whereas mating and birthing in the Gulf of Mexico take place in late spring or early summer (May to August).[20][32] However, in some cases, the presence of reproductive seasonality may have been obscured by biases in data collection.[3] Females give birth after a gestation period of 12 months, either every year or every other year.[1] The litter size ranges from one to 16 and increases with female size, with six to 12 being typical.[3] The pups are born in reef nursery areas on the outer continental shelf, where ample food supplies and protection from large pelagic sharks occur. The risk of predation has selected for fast growth in young sharks, which add 25–30 cm (9.8–11.8 in) to their length within their first year of life. After a few months (or by the first winter in the Gulf of Mexico), the now-subadult sharks migrate out from the nursery into the open ocean.[3][30][32]
The life history characteristics of the silky shark differ across its range (see table). Northwestern Atlantic sharks tend to be larger than those in the western-central Pacific at all ages, while eastern Pacific sharks tend to be smaller than sharks in other regions. Eastern Atlantic and Indian Ocean sharks seem to match or exceed the size of northwestern Atlantic sharks, but the figures are based on relatively few individuals and more data are needed.[3]
The overall growth rate of the silky shark is moderate compared to other shark species and similar for both sexes, though it varies significantly between individuals. One central Pacific study has found females growing much slower than males, but the results may have been skewed by missing data from large females.[20] The highest reported growth rates are from sharks in the northern Gulf of Mexico, and the lowest from sharks off northeastern Taiwan.[47] Males and females reach sexual maturity at ages of 6–10 years and 7–12+ years, respectively.[3] Sharks from more temperate waters may grow slower and mature later than those in warmer regions.[47] The maximum lifespan is at least 22 years.[27]
Given its formidable size and dentition, the silky shark is regarded as potentially dangerous to humans. However, it only rarely comes into contact with people due to its oceanic habits.[8] Its natural curiosity and boldness may lead it to repeatedly and closely approach divers, and it can become dangerously excited in the presence of food. The silky shark tends to be more aggressive if encountered on a reef than in open water. Cases of individual sharks persistently harassing divers and even forcing them out of the water have been reported.[39][49] As of May 2009, the International Shark Attack File lists six attacks attributable to the silky shark, three of them unprovoked and none fatal.[50]
Large numbers of silky sharks are caught by commercial and artisanal multispecies shark fisheries operating off Mexico, Guatemala, El Salvador, Costa Rica, the United States, Ecuador, Spain, Portugal, Sri Lanka, the Maldives, Yemen, and Côte d'Ivoire. Even greater numbers are caught incidentally by tuna longline and purse seine fisheries throughout its range, particularly those using fish aggregating devices. It is the most common shark caught as bycatch in the eastern Pacific and Gulf of Mexico tuna fisheries, and the second-most common shark caught as bycatch (next to the blue shark) overall.[3][51] The fins are valued as an ingredient in shark fin soup, with captured sharks often finned at sea and the rest of the body discarded. Fins from an estimated one-half to one and a half million silky sharks are traded globally per year; it is the second- or third-most common species auctioned on the Hong Kong fin market, which represents over half the global trade.[1][3] The meat (sold fresh or dried and salted), skin, and liver oil may also be used,[5] as well as the jaws: this species is the predominant source of dried shark jaw curios sold to tourists in the tropics.[30] Some sport fishers catch silky sharks.[8]
As one of the most abundant and widely distributed sharks on Earth, the silky shark was once thought to be mostly immune to depletion despite heavy fishing mortality. In 1989 alone, some 900,000 individuals were taken as bycatch in the southern and central Pacific tuna longline fishery, seemingly without effect on the total population.[27] Fishery data on this shark are often confounded by under-reporting, lack of species-level separation, and problematic identification. Nevertheless, mounting evidence indicates the silky shark has, in fact, declined substantially worldwide, a consequence of its modest reproductive rate which is unable to sustain such high levels of exploitation. The total annual catch reported to the Food and Agriculture Organization fell steadily from 11,680 tons in 2000 to 4,358 tons in 2004. Regional assessments have found similar trends, estimating declines of some 90% in the central Pacific from the 1950s to the 1990s, 60% off Costa Rica from 1991 to 2000, 91% in the Gulf of Mexico from the 1950s to the 1990s, and 85% (for all large requiem sharks) in the northwestern Atlantic from 1986 to 2005. The silky shark fishery off Sri Lanka reported a drop from a peak catch of 25,400 tons in 1994 to only 1,960 tons in 2006, indicative of a local stock collapse. However, Japanese fisheries in the Pacific and Indian Oceans have recorded no change in catch rate between the 1970s and the 1990s,[1] and the validity of the methodologies used to assess declines in the Gulf of Mexico and the northwestern Atlantic have come under much debate.[52][53][54]
As of 2017, the silky shark is classified by the International Union for Conservation of Nature as a vulnerable species. The silky shark is listed on Annex I, Highly Migratory Species, of the United Nations Convention on the Law of the Sea, though this has yet to result in any management schemes. The species should benefit from bans on shark finning, which are being increasingly implemented by nations and supranational entities, including the United States, Australia, and the European Union.[1] Organizations such as the International Commission for the Conservation of Atlantic Tunas and the Inter-American Tropical Tuna Commission have also taken steps to improve fishery monitoring, with the ultimate goal of reducing shark bycatch.[3] However, given the highly migratory nature of the silky shark and its association with tuna, no simple way is known to reduce bycatch without also affecting the economics of the fishery.[23]
{{cite journal}}: CS1 maint: multiple names: authors list (link) {{cite book}}: CS1 maint: multiple names: authors list (link) The silky shark (Carcharhinus falciformis), also known by numerous names such as blackspot shark, gray whaler shark, olive shark, ridgeback shark, sickle shark, sickle-shaped shark and sickle silk shark, is a species of requiem shark, in the family Carcharhinidae, named for the smooth texture of its skin. It is one of the most abundant sharks in the pelagic zone, and can be found around the world in tropical waters. Highly mobile and migratory, this shark is most often found over the edge of the continental shelf down to 50 m (164 ft). The silky shark has a slender, streamlined body and typically grows to a length of 2.5 m (8 ft 2 in). It can be distinguished from other large requiem sharks by its relatively small first dorsal fin with a curving rear margin, its tiny second dorsal fin with a long free rear tip, and its long, sickle-shaped pectoral fins. It is a deep, metallic bronze-gray above and white below.
With prey often scarce in its oceanic environment, the silky shark is a swift, inquisitive, and persistent hunter. It feeds mainly on bony fishes and cephalopods, and has been known to drive them into compacted schools before launching open-mouthed, slashing attacks. This species often trails schools of tuna, a favored prey. Its sense of hearing is extremely acute, allowing it to localize the low-frequency noises generated by other feeding animals, and, by extension, sources of food. The silky shark is viviparous, meaning that the developing embryos are sustained by a placental connection to their mother. Significant geographical variation is seen in its life history details. Reproduction occurs year-round except in the Gulf of Mexico, where it follows a seasonal cycle. Females give birth to litters of up to 16 pups annually or biennially. The newborn sharks spend their first months in relatively sheltered reef nurseries on the outer continental shelf, growing substantially before moving into the open ocean.
The large size and cutting teeth of the silky shark make it potentially dangerous, and it has behaved aggressively towards divers. However, attacks are rare, as few humans enter its oceanic habitat. Silky sharks are valued for their fins, and to a lesser extent their meat, hide, liver oil, and jaws. Because of their abundance, they form a major component of commercial and artisanal shark fisheries in many countries. Furthermore, their association with tuna results in many sharks being taken as bycatch in tuna fisheries. Although slow-reproducing like most other sharks, the wide distribution and large population size of the silky shark was once thought to buffer the species against these fishing pressures. However, data now suggest that silky shark numbers are declining around the world, which prompted the IUCN to reassess its conservation status to Vulnerable in 2017.
El tiburón sedoso (Carcharhinus falciformis) es una especie de elasmobranquio carcarriniforme de la familia Carcharhinidae[1] que habita latitudes intertropicales.
Carcharhinus faciformis es gris oscuro con reflejos bronceados en el dorso y blanco en el vientre. Las puntas de las aletas dorsales son más oscuras que el resto del cuerpo.
Aunque fundamentalmente pelágico, el tiburón sedoso no se limita a mar abierto y se han registrado casos de avistamientos en profundidades de 18 metros. Se trata de un tiburón muy activo y rápido que prefiere las aguas cálidas (23 °C). Es frecuente encontrarlos cerca de los bordes de plataformas continentales y en los arrecifes de aguas profundas, abundante fuente de alimento. Normalmente, nada hasta profundidades de 500 m, pero se han recogido datos de ejemplares a más profundidad. Este tiburón presenta segregación sexual, que se refleja en el hábito de viajar con congéneres del mismo tamaño.
El tiburón sedoso sudoroso es un tiburón común de las zonas tropicales, subterráneas y subtropicales del océano Atlántico, Pacífico e Índico. En el Atlántico occidental, se distribuye desde Massachusetts a Brasil (incluyendo el Golfo de México y Mar Caribe) y de España a Angola en el Atlántico oriental. En el Océano Índico occidental se puede encontrar en el Mar Rojo y desde Tanzania a Mozambique, incluyendo Madagascar y Comoras, y en el Índico oriental desde las Maldivas y Sri Lanka a Australia Occidental. Se encuentra también de China a Nueva Zelanda en el Pacífico occidental (incluyendo las islas hawaianas), y desde California hasta Chile en el Pacífico Oriental. Es muy común en la Isla Malpelo(Colombia), pero muchas poblaciones se ven amenazadas por el aleteo de tiburones.[2]
El tiburón sedoso (Carcharhinus falciformis) es una especie de elasmobranquio carcarriniforme de la familia Carcharhinidae que habita latitudes intertropicales.
Carcharhinus falciformis Carcharhinus generoko animalia da. Arrainen barruko Carcharhinidae familian sailkatzen da.
Carcharhinus falciformis Carcharhinus generoko animalia da. Arrainen barruko Carcharhinidae familian sailkatzen da.
Carcharhinus falciformis
Le Requin soyeux (Carcharhinus falciformis) est une espèce de requins de la famille des Carcharhinidae, qui doit son nom à la texture lisse de sa peau. Il est l'un des requins les plus abondants dans la zone pélagique, et peut être trouvé dans les océans tropicaux du monde entier. Très mobile et migrateur, ce requin vit le plus souvent sur le bord du plateau continental jusqu'à une profondeur de 50 mètres. Le Requin soyeux a un corps mince et fuselé et atteint généralement une longueur de 2,5 mètres. Il se distingue des autres grands requins par sa première nageoire dorsale de petite taille avec une marge postérieure courbe, sa petite deuxième nageoire dorsale avec une longue pointe arrière libre et ses longues nageoires pectorales en forme de faucille. Il est d'un profond gris bronze métallisé au-dessus et blanc au-dessous.
Les proies étant souvent rares dans son environnement océanique, le Requin soyeux est un chasseur rapide, curieux et insistant. Il se nourrit principalement de poissons osseux et de céphalopodes, et est connu pour les chasser en les regroupant en des bancs compacts avant de se jeter dedans la gueule ouverte. Cette espèce suit souvent les bancs de thons, une de ses proies privilégiées. Son ouïe est extrêmement développée, ce qui lui permet de localiser les sons à de basses fréquences, générés par les autres animaux et, par extension, les sources de nourriture. Le Requin soyeux est vivipare, ce qui signifie que les embryons en développement sont reliés au placenta de leur mère. Son cycle de vie varie fortement suivant les régions. La reproduction a lieu toute l'année, sauf dans le golfe du Mexique, où elle suit un cycle saisonnier. Les femelles donnent naissance à des portées allant jusqu'à 16 jeunes par an — ou tous les deux ans. Les requins nouveau-nés passent leurs premiers mois dans des zones de récifs relativement protégées sur le plateau continental, où ils grandissent, avant de gagner les eaux libres océaniques.
Du fait de sa grande taille et de ses dents acérées, le Requin soyeux est potentiellement dangereux pour l'homme, car il peut avoir un comportement agressif envers les plongeurs. Cependant, les attaques sont rares, car peu d'humains pénètrent dans son habitat océanique. En revanche, l'espèce est pêchée pour ses ailerons et, dans une moindre mesure, pour sa viande, sa peau, son huile de foie et ses mâchoires. En raison de son abondance, ce poisson est une cible de choix pour les pêcheurs de requins de nombreux pays. Par ailleurs, comme il est souvent associé aux bancs de thons, il constitue une prise accessoire des pêcheurs. En dépit de son faible taux de reproduction, il semble que son importante aire de répartition et sa grande population permettent d'atténuer la baisse des effectifs liée à la pêche.[réf. souhaitée] Toutefois, les dernières données recueillies[réf. souhaitée] sur cet animal indiquent que le nombre de Requins soyeux est en baisse partout dans le monde, ce qui a incité l'Union internationale pour la conservation de la nature (UICN) à réévaluer son statut de conservation de « préoccupation mineure » à « quasi-menacé » en 2007.
La première description scientifique du Requin soyeux a été publiée par les biologistes allemands Johannes Müller et Jacob Henle sous le nom de Carcharias (Prionodon) falciformis, dans leur Systematische Beschreibung der Plagiostomen de 1839, à partir d'une description communiquée par Gabriel Bibron[1]. Des auteurs ultérieurs ont ensuite placé cette espèce dans le genre Carcharhinus[2],[3],[4]. Comme le spécimen type de Müller et Henle était un fœtus femelle de 53 cm de long capturé à Cuba, les premiers adultes rencontrés ne sont pas reconnus comme C. falciformis et sont décrits comme une espèce distincte, Carcharhinus floridanus, par Henry Bigelow, William Schroeder et Stewart Springer en 1943. Jack Garrick, Richard Backus et Robert Gibbs, Jr. placent C. floridanus comme un synonyme de C. falciformis en 1964[5].
L'épithète spécifique falciformis signifie en latin « en forme de faucille », en référence au profil des nageoires pectorales et dorsales de cette espèce[6]. Le nom commun du Requin soyeux provient de sa peau qui a une texture fine par rapport aux autres requins, du fait de ses minuscules denticules cutanés densément implantés[7].
Le Requin soyeux a un corps mince et fuselé, avec un assez long museau arrondi qui présente des rabats de peau à peine développés devant les narines. Les yeux sont circulaires et de taille moyenne, et sont équipés de membranes nictitantes. Des sillons courts et peu profonds sont présents au niveau des coins de la bouche[3],[8]. Il y a entre 14 et 16 et 13 et 17 rangées de dents de chaque côté des mâchoires supérieure et inférieure respectivement (en général 15 pour les deux). Les dents de la mâchoire supérieure sont triangulaires et fortement dentelées, avec une encoche dans le bord postérieur. Elles sont droites au centre et deviennent plus obliques vers les côtés. Les dents de la mâchoire inférieure sont étroites, droites et lisses. Les cinq paires de fentes branchiales sont de longueur modérée[9].
Les nageoires dorsales et pectorales sont caractéristiques et aident à distinguer le Requin soyeux des espèces similaires. La première nageoire dorsale est relativement petite, mesurant moins d'un dixième de la longueur du requin, et elle prend naissance derrière les extrémités arrière libres des nageoires pectorales. Elle a un sommet arrondi, une bordure arrière en forme de « S » et une pointe arrière libre mesurant environ la moitié de la longueur de la nageoire. La deuxième nageoire dorsale est petite, plus petite même que la nageoire anale, avec une très longue extrémité libre à l'arrière, jusqu'à trois fois plus longue que la nageoire. Il y a une crête étroite s'étendant entre les nageoires dorsales. Les nageoires pectorales sont étroites et falciformes, et particulièrement longues chez les adultes. La nageoire anale est implantée légèrement avant la deuxième nageoire dorsale et a une profonde entaille dans le bord postérieur. La nageoire caudale est assez haute avec un lobe inférieur bien développé[3],[8].
La peau est densément couverte par des denticules cutanés qui se chevauchent. Chaque denticule cutané est en forme de diamant et porte des nervures horizontales se terminant par des dentelures postérieures marginales, qui sont de plus en plus nombreuses au fur et à mesure que le requin se développe[5],[7]. Le dos est gris-doré métallisé foncé et le ventre est d'un blanc neigeux qui s'étend sur le flanc en une bande claire. Les nageoires (à l'exception de la première dorsale) s'assombrissent à leur extrémité, ce qui est plus visible chez les jeunes requins[3],[7]. La coloration s'estompe rapidement pour donner un gris terne après la mort de l'animal[10]. Le Requin soyeux est l'un des plus grands membres de son genre. Il atteint généralement une longueur de 2,5 m[11], avec une longueur maximale enregistrée de 3,5 m[11] et un poids de 346 kg[12]. Les femelles sont plus grandes que les mâles[7].
Le Requin soyeux est l'une des trois espèces de requins pélagiques les plus communes, avec le Requin bleu et le Requin longimane, et il compte parmi les grands animaux les plus abondants des océans du monde, avec une population d'au moins plusieurs dizaines de millions d'individus[13]. Par rapport aux deux autres espèces, il est moins strictement associé à la zone pélagique, et on le trouve souvent dans des zones côtières, où la nourriture est plus facile à obtenir que plus loin dans l'océan. Le Requin soyeux est un prédateur actif, curieux et agressif[11], mais qui est dominé par le plus lent mais plus puissant Requin longimane quand ces deux espèces se retrouvent en concurrence[3]. À l'approche de quelque chose d'intéressant, il peut sembler inattentif, décrivant des cercles calmement et balançant parfois sa tête de gauche à droite. Cependant, il peut répondre avec une rapidité surprenante à tout changement dans son environnement immédiat[14]. On trouve souvent ce requin autour d'objets flottants tels que des bouts de bois ou des bouées nautiques[15].
Les jeunes Requins soyeux sont connus pour former de grands groupes plus ou moins organisés, peut-être pour se défendre mutuellement[16]. Pendant les migrations, plus de mille individus peuvent se rassembler[17]. Ces groupes sont généralement constitués d'animaux de même taille, et dans le Pacifique peut-être aussi suivant le sexe[7],[18],[19]. Les Requins soyeux au sein d'un groupe sont souvent observés se présentant leur profil latéral l'un l'autre, ou à entrouvrir leurs mâchoires ou gonfler leurs branchies. À l'occasion, les requins ont également été vu chargeant soudainement vers le haut, virant juste avant d'atteindre la surface pour retourner vers le bas et les eaux plus profondes. La signification de ces comportements est inconnue[14]. Lorsqu'il est confronté à une menace, le Requin soyeux peut prendre une posture d'intimidation, dans laquelle il cambre son dos, baisse sa queue et ses nageoires pectorales et lève la tête. Le requin nage alors en formant des boucles serrées dans un mouvement raide et saccadé, en se retournant souvent vers la menace perçue[20].
Les prédateurs du Requin soyeux sont les grands requins et les orques (Orcinus orca). Parmi les parasites qui lui sont connus, on compte l'isopode Gnathia trimaculata[21], le copépode Kroeyerina cortezensis[22] et les cestodes Dasyrhynchus variouncinatus et Phyllobothrium[23],[24]. Les Requins soyeux se mêlent souvent aux bancs de Requins-marteaux halicornes (Sphyrna lewini), et sont connus pour suivre les mammifères marins. Un recensement dans la mer Rouge a décrit 25 Requins soyeux suivant un grand groupe de dauphins Tursiops, avec 25 Requins gris de récif (C. amblyrhynchos) et un Requin pointe blanche (C. albimarginatus). Les Requins soyeux sont eux-mêmes accompagnés de jeunes Poissons-pilotes (Naucratès ductor), ainsi que par des Carangidae, qui se nourrissent de restes de nourriture et viennent se frotter contre la peau du requin pour se débarrasser de leurs parasites[16],[25].

Le Requin soyeux est un prédateur opportuniste se nourrissant principalement de poissons osseux dans tous les niveaux de la colonne d'eau, dont notamment les thons, les maquereaux, les sardines, les rougets, les mérous, les Lutjanidae, les Decapterus, les Kyphosidae, les poissons-chats marins, les anguilles, les poissons-lanternes, les Monacanthidae, les balistes et les Diodontidae. Il peut également consommer des calmars, des argonautes et des Portunidae, et il existe des preuves fossiles que ce requin peut venir se nourrir sur des carcasses de baleines[6],[3],[26]. De fortes disponibilités en nourriture de qualité peuvent attirer les Requins soyeux en grand nombre. Un tel groupe de requin a été observé piégeant un banc de poissons en l'encerclant près de la surface de l'eau, le regroupant en une masse compacte avant que les requins ne consomment l'intégralité du banc[6]. Lors d'une attaque d'un banc de poissons dense, le Requin soyeux charge à travers le banc la bouche ouverte, et capture des poissons dans les coins de sa mâchoire. Bien que plusieurs requins peuvent se nourrir à la fois, chacun lance son attaque de façon indépendante[16].
Des études menées au large de la côte de la Floride et des Bahamas ont montré que les Requins soyeux sont très sensibles au son, en particulier à ceux de basse fréquence (10 à 20 Hz) et aux impulsions irrégulières. Des expériences dans lesquelles ce genre de sons ont été joués sous l'eau ont permis d'attirer des requins venant parfois de plusieurs centaines de mètres. Les Requins soyeux s'orientent vers la source de ces sons car ils sont semblables à ceux générés par l'alimentation d'animaux comme les oiseaux ou les dauphins, et indiquent donc des sources de nourriture[14],[16]. Ces études ont également démontré que le Requin soyeux attiré par un son va rapidement se retirer si ce son change brusquement en amplitude ou en caractère ; ce changement ne doit pas forcément être un son produit par un prédateur pour engendrer une réaction immédiate. Au fil des expositions répétées, les Requins soyeux s'habituent au changement de son et arrêtent de se retirer, mais il leur faut plus de temps pour modifier leur comportement que pour le Requin longimane, plus audacieux[27].
La force d'une morsure d'un requin de 2 m de long a été mesurée à 890 newtons[28]. Il existe une association bien établie entre cette espèce et le thon. Au large du Ghana, presque tous les bancs de thons sont suivis de Requins soyeux, et dans l'est du Pacifique ces requins infligent de tels dommages aux filets de pêche au thon et aux captures que les pêcheurs de thon le surnomment parfois « requin mangeur de filet »[3],[10]. Les Requins soyeux et les grands dauphins sont en concurrence lorsque les deux espèces ciblent le même banc de poisson, et la quantité consommée par les dauphins diminue quand le nombre de requins présents augmente. S'il y a un grand nombre de requins, ils ont tendance à rester à l'intérieur du banc des proies, tandis que les dauphins se confinent à la périphérie, peut-être pour éviter les blessures accidentelles liées aux attaques de requins. Inversement, si un assez grand groupe de dauphins se rassemble, ils deviennent capables de chasser les requins du banc de proies. Peu importe où l'on domine, les deux prédateurs ne s'engagent pas dans un comportement ouvertement agressif l'un contre l'autre[29].
Comme les autres membres de sa famille, le Requin soyeux est vivipare : une fois que l'embryon en développement épuise son approvisionnement en vitellus, le sac vitellin appauvri est converti en une connexion placentaire par lequel la mère fournit la nourriture. Par rapport aux autres requins vivipares, le placenta du Requin soyeux est moins similaire à la structure analogue des mammifères car il n'y a pas d'entrecroisement entre les tissus du fœtus et la mère. De plus, les globules rouges fœtaux sont beaucoup plus petits que les globules maternels, ce qui va à l'inverse de la tendance observée chez les mammifères. Les femelles adultes ont un seul ovaire fonctionnel (sur le côté droit) et deux utérus, qui sont divisés en longueur en des compartiments séparés pour chaque embryon[30].
Les Requins soyeux dans la plupart des régions du monde se reproduisant apparemment toute l'année, alors que l'accouplement et la naissance ont lieu dans le Golfe du Mexique à la fin du printemps ou au début de l'été (de mai à août)[31],[19]. Cependant, dans certains cas, la présence d'une saisonnalité de la période de reproduction peut avoir été obscurcie par des biais dans la collecte des données[6]. Les femelles donnent naissance après une période de gestation de 12 mois, soit chaque année ou tous les deux ans[32]. La taille de la portée varie de 1 à 16 et augmente en fonction de la taille de la femelle, un nombre de 6 à 12 étant typique[11],[6]. Les jeunes sont nés dans les zones de reproduction des récifs, sur le plateau continental, là où les approvisionnements alimentaires sont suffisants et la protection contre les grands requins pélagiques assurée. Le risque de prédation a sélectionné cette espèce sur une croissance rapide des jeunes requins, qui prennent 25 à 30 cm au cours de leur première année de vie. Après quelques mois (ou pendant le premier hiver dans le Golfe du Mexique), les requins subadultes migrent des nurseries côtières vers les eaux libres de l'océan[6],[16],[19].
Les caractéristiques du cycle de vie du Requin soyeux diffèrent suivant la zone géographique. Les requins de l'Atlantique du Nord-Ouest ont tendance à être plus grands que ceux du Pacifique centre-ouest à tout âge, tandis que les requins du Pacifique Est ont tendance à être plus petits que les requins des autres régions. Les requins de l'Atlantique Est et de l'océan Indien semblent égaler ou dépasser la taille des requins de l'Atlantique nord-ouest, mais les chiffres sont basés sur relativement peu d'individus et plus de données sont nécessaires[6].
Le taux de croissance moyen du Requin soyeux est modéré par rapport à celui d'autres espèces de requins, et il est similaire quel que soit le sexe, même s'il varie considérablement d'un individu à l'autre. Une étude dans le centre du Pacifique a montré que les femelles grandissaient moins vite que les mâles, mais les résultats ont été faussés par l'absence de données sur de grandes femelles[31]. Les croissances les plus élevées semblent obtenues par les requins du nord du Golfe du Mexique, et les plus basses par les requins vivant au large du nord de Taïwan[37]. Les mâles et les femelles atteignent la maturité sexuelle à l'âge de 6 à 10 ans et 7-12 ans respectivement[6]. Les requins des eaux plus tempérées peuvent croître plus lentement et arriver à maturité plus tard que dans les régions plus chaudes[37]. La longévité maximale est d'au moins 22 ans[13].
Le Requin soyeux a une répartition cosmopolite dans les eaux marines aux températures dépassant 23 °C. Dans l'océan Atlantique, on le rencontre de l’État américain du Massachusetts jusqu'à l'Espagne au nord, et du sud du Brésil jusqu'au nord de l'Angola au sud, en passant par la mer Méditerranée, le Golfe du Mexique, et la mer des Caraïbes. Il vit dans l'ensemble de l'océan Indien, au sud jusqu'au Mozambique à l'ouest et jusqu'à l'Australie-Occidentale à l'Est, en passant par la mer Rouge et le Golfe Persique. Dans l'océan Pacifique, la limite nord de son aire de répartition s'étend du sud de la Chine et du Japon jusqu'au sud de la péninsule de Basse-Californie et au Golfe de Californie, tandis que la limite sud va de Sydney, en Australie, et du nord de la Nouvelle-Zélande jusqu'au nord du Chili[6],[3]. Si on s'appuie sur les différences visibles au niveau de leur cycle de vie, quatre populations distinctes de Requins soyeux ont été identifiées dans les bassins océaniques du monde entier : une dans l'Atlantique nord-ouest, une dans le Pacifique occidental et central, une dans le Pacifique oriental et une dans l'océan Indien[6].
On a observé des requins soyeux se regroupant autour des centrales électriques en Méditerranée orientale et plus précisément dans les eaux israéliennes de Hadera[39] .
Principalement résident de la zone pélagique, le Requin soyeux est généralement présent entre la surface et une profondeur de 200 m, mais peut plonger jusqu'à 500 m ou plus[3]. Les suivis d'individus marqués dans la zone tropicale orientale du Pacifique et au nord du Golfe du Mexique ont constaté que les Requins soyeux passent 99 % de leur temps à moins de 50 m de profondeur, et 80 à 85 % de leur temps dans une eau à une température comprise entre 26 et 30 °C, et ce de jour comme de nuit[40],[41]. Cette espèce préfère les bords des plateaux continentaux et insulaires, souvent au-dessus de récifs d'eaux profondes et autour des îles. Son aire de répartition est plus étendue vers le nord et le sud le long des marges continentales que dans les eaux libres océaniques. À l'occasion, il peut s'aventurer dans les eaux côtières peu profondes, jusqu'à moins de 18 m de fond[31]. Les Requins soyeux sont très mobiles et migrateurs, bien que les détails de leurs déplacements sont peu connus. Les données de marquage ont enregistré des requins parcourant jusqu'à 60 km par jour, et couvrant des distances pouvant atteindre 1 339 km[42]. Les grands requins se déplacent généralement sur de plus longues distances que les plus petits. Dans l'océan Pacifique et peut-être ailleurs, ils passent l'été à des latitudes un peu plus élevées, en particulier pendant les années les plus chaudes d'El Niño[18],[43]. Dans l'Atlantique Nord, la plupart des requins suivent le Gulf Stream vers le nord le long de la côte Est des États-Unis[42]. Dans le Golfe d'Aden, il est plus fréquent en fin de printemps et d'été[6].
Carcharhinus falciformis
Des dents fossilisées appartenant au Requin soyeux ont été découvertes en Caroline du Nord : une dans de la boue datant du Pléistocène - Holocène (il y a 12 000 ans), une autre dans une formation calcaire de Goose Creek datant du Pliocène (il y a 3,5 millions d'années), ainsi que dans la rivière Pungo, datant du Miocène (de 23 à 5,3 millions d'années avant notre ères)[26],[45]. Des fossiles de dents ont également été trouvés dans les strates du Pliocène de la carrière Cava Serredi en Toscane, en Italie[46]. Carcharhinus elongatus, un ancêtre de sa lignée portant des dents lisses et tranchantes, vivait à l'Oligocène (il y a entre 34 et 23 millions d'années) dépôts dans la formation vieille église de la Virginie, et la formation Ashley de Caroline du Sud. Un ensemble de dents mal décrites datant de l'Éocène (il y a entre 56 et 34 millions d'années) et ressemblant à celles de cette espèce ont été découvertes en Égypte[45].
Les premiers efforts pour comprendre les relations évolutives du Requin soyeux n'ont pas été concluants. S'appuyant sur la morphologie des animaux, Jack Garrick suggère en 1982 que le Requin à taches noires (C. sealei) est son plus proche parent[47]. En 1988, Leonard Compagno le place dans un groupe informel avec le Requin nez noir (C. acronotus), le Requin à pointes noires (C. melanopterus), le Requin nerveux (C. cautus), le Requin cuivre (C. brachyurus) et le Requin de nuit (C. signatus)[48].
Plus récemment, l'analyse phylogénétique de 1992 menée par Gavin Naylor, sur la base de données de séquences allozymes, a constaté que le Requin soyeux faisait partie d'un groupe comprenant des grands requins portant une crête entre les nageoires dorsales. Une branche de ce groupe contient le Requin gris (C. plumbeus) et le Requin babosse (C. altimus), tandis que le Requin soyeux est le membre de base de l'autre branche et un taxon frère à un clade comprenant le Requin de récif (C. perezi), le Requin des Galápagos (C. galapagensis), le Requin longimane (C. longimanus), le Requin requiem de sable (C. obscurus), et le Requin bleu (Prionace glauca)[44]. L'analyse de l'ARN ribosomique réalisée par Mine Dosay-Abkulut en 2008, a confirmé que le Requin soyeux était proche du Requin babosse et du Requin bleu[49].
Compte tenu de sa taille et de sa formidable dentition, le Requin soyeux est considéré comme dangereux pour l'Homme. Cependant, il ne vient que rarement en contact avec des humains en raison de son habitat principalement océanique[7]. Sa curiosité naturelle et son audace peuvent le conduire à approcher les plongeurs, et il peut alors devenir dangereux s'il est excité par la présence de nourriture[50]. Le Requin soyeux a tendance à être plus agressif si on le rencontre sur un récif plutôt qu'en eau libre. Il y a quelques cas recensés de requins harcelant constamment des plongeurs et les forçant même à sortir hors de l'eau[25],[51]. En mai 2009, l'International Shark Attack File recense six attaques imputables au Requin soyeux, trois d'entre elles provoquées par les victimes et aucune n'ayant été mortelle[52].
Un grand nombre de Requins soyeux sont capturés par les pêcheurs de requins artisanaux et commerciaux, opérant au large du Mexique, du Guatemala, du Salvador, du Costa Rica, des États-Unis, de l'Équateur, de l'Espagne, du Portugal, du Sri Lanka, des Maldives, du Yémen ou de la Côte d'Ivoire. Un nombre encore plus important est capturé accessoirement par les pêcheurs de thon à la palangre ou au filet dérivant[53], en particulier par les ceux utilisant des dispositifs de concentration de poissons (DCP). C'est le requin le plus communément pris accidentellement dans l'est du Pacifique et dans le Golfe du Mexique par les pêcheurs de thon, et le deuxième requin le plus communément capturé comme prise accessoire toutes pêches confondues, derrière le Requin bleu[6],[54]. Ainsi par exemple 3 353 tonnes de requins soyeux contre 9 540 tonnes de requins peau bleue ont été déclarés pêchées en 2011 selon la Commission des thons de l'océan Indien[55]. Ces ailerons sont utilisés pour confectionner la soupe d'ailerons de requin, et la pratique du shark finning est courante avec cette espèce. Au total ce sont les ailerons d'entre un demi-million à un million et demi de Requins soyeux qui approvisionnent le marché mondial, et il s'agit de la seconde ou troisième espèce la plus vendue sur le marché des ailerons de Hong Kong, qui représente environ la moitié du marché mondial[32],[6]. On valorise sa viande, vendue fraîche ou séchée et salée, sa peau et son huile de foie[3], ainsi que ses mâchoires ; cette espèce constitue le principal pourvoyeur de mâchoires vendues comme curiosités pour les touristes dans les tropiques[16]. Certains pêcheurs sportifs capturent des Requins soyeux[7].
Comme il s'agit de l'un des requins les plus abondants et les plus répandus sur Terre, on a longtemps pensé que le Requin soyeux était à l'abri du déclin de sa population, malgré la forte mortalité liée à la pêche. En 1989, quelque 900 000 individus ont été capturés de façon accessoire par la pêche à la palangre du thon dans le centre et le sud du Pacifique sud, apparemment sans effet sur la population totale[13]. Les données de la pêche de ce requin sont faussées par les fréquentes sous-déclarations et les problèmes d'identification de l'espèce. Néanmoins, il y a des preuves croissantes que la population mondiale de Requin soyeux diminue de manière substantielle, conséquence de son taux de reproduction modeste qui est incapable de soutenir les niveaux élevés de capture. La capture annuelle totale déclarée à l'Organisation pour l'alimentation et l'agriculture (FAO) a régulièrement diminué, passant de 11 680 tonnes en 2000 à 4 358 tonnes en 2004. Les évaluations régionales ont constaté des tendances similaires, le déclin de la population pourrait ainsi atteindre 90% dans le Pacifique central entre les années 1950 et les années 1990, 60 % au large du Costa Rica entre 1991 à 2000, 91 % dans le Golfe du Mexique des années 1950 aux années 1990, et 85 % (comme pour tous les grands requins de la famille des Carcharhinidae) dans l'Atlantique Nord-Ouest entre 1986 et 2005. La pêche au Requin soyeux au large du Sri Lanka a enregistré une baisse entre le pic de 25 400 tonnes en 1994 et les 1 960 tonnes de 2006, ce qui indique un effondrement de la population locale. Par contre, les pêcheurs japonais dans le Pacifique et l'océan Indien ont enregistré aucun changement dans le taux de capture entre les années 1970 et 1990[32], et la validité des méthodes utilisées pour évaluer la baisse des effectifs dans le Golfe du Mexique et de l'Atlantique nord-ouest a fait l'objet de nombreux débats[56],[57],[58].
À la suite des découvertes récentes, l'Union internationale pour la conservation de la nature (UICN) a réévalué en 2007 le statut du Requin soyeux, le passant de préoccupation mineure à quasi-menacé. Au niveau régional, il est répertorié comme quasi-menacé dans le sud-ouest de l'Atlantique, l'océan Indien et le centre-ouest du Pacifique, et comme vulnérable dans la partie orientale du Pacifique centre et sud et le nord-ouest et l'ouest de l'océan Atlantique. Le Requin soyeux est inscrit à l'Annexe I, avec les espèces de grands migrateurs, sur la Convention des Nations unies sur le droit de la mer, bien que cela n'ait pas encore entraîné la mise en place de schémas de sauvegarde. L'espèce devrait bénéficier de l'interdiction du shark finning, qui est de plus en plus mise en œuvre par les pays et les entités supranationales, y compris les États-Unis, l'Australie et l'Union européenne[32]. Des organisations telles que la Commission internationale pour la conservation des thonidés de l'Atlantique (CICTA) et le Inter-American Tropical Tuna Commission (IATTC) ont également pris des mesures pour améliorer la surveillance de la pêche, dans le but de réduire les prises accessoires de requins[6]. Cependant, étant donné le caractère hautement migratoire du Requin soyeux et son association avec le thon, il n'existe aucun moyen simple de réduire les prises accessoires sans affecter aussi l'économie de la pêche[43].
Carcharhinus falciformis
Le Requin soyeux (Carcharhinus falciformis) est une espèce de requins de la famille des Carcharhinidae, qui doit son nom à la texture lisse de sa peau. Il est l'un des requins les plus abondants dans la zone pélagique, et peut être trouvé dans les océans tropicaux du monde entier. Très mobile et migrateur, ce requin vit le plus souvent sur le bord du plateau continental jusqu'à une profondeur de 50 mètres. Le Requin soyeux a un corps mince et fuselé et atteint généralement une longueur de 2,5 mètres. Il se distingue des autres grands requins par sa première nageoire dorsale de petite taille avec une marge postérieure courbe, sa petite deuxième nageoire dorsale avec une longue pointe arrière libre et ses longues nageoires pectorales en forme de faucille. Il est d'un profond gris bronze métallisé au-dessus et blanc au-dessous.
Les proies étant souvent rares dans son environnement océanique, le Requin soyeux est un chasseur rapide, curieux et insistant. Il se nourrit principalement de poissons osseux et de céphalopodes, et est connu pour les chasser en les regroupant en des bancs compacts avant de se jeter dedans la gueule ouverte. Cette espèce suit souvent les bancs de thons, une de ses proies privilégiées. Son ouïe est extrêmement développée, ce qui lui permet de localiser les sons à de basses fréquences, générés par les autres animaux et, par extension, les sources de nourriture. Le Requin soyeux est vivipare, ce qui signifie que les embryons en développement sont reliés au placenta de leur mère. Son cycle de vie varie fortement suivant les régions. La reproduction a lieu toute l'année, sauf dans le golfe du Mexique, où elle suit un cycle saisonnier. Les femelles donnent naissance à des portées allant jusqu'à 16 jeunes par an — ou tous les deux ans. Les requins nouveau-nés passent leurs premiers mois dans des zones de récifs relativement protégées sur le plateau continental, où ils grandissent, avant de gagner les eaux libres océaniques.
Du fait de sa grande taille et de ses dents acérées, le Requin soyeux est potentiellement dangereux pour l'homme, car il peut avoir un comportement agressif envers les plongeurs. Cependant, les attaques sont rares, car peu d'humains pénètrent dans son habitat océanique. En revanche, l'espèce est pêchée pour ses ailerons et, dans une moindre mesure, pour sa viande, sa peau, son huile de foie et ses mâchoires. En raison de son abondance, ce poisson est une cible de choix pour les pêcheurs de requins de nombreux pays. Par ailleurs, comme il est souvent associé aux bancs de thons, il constitue une prise accessoire des pêcheurs. En dépit de son faible taux de reproduction, il semble que son importante aire de répartition et sa grande population permettent d'atténuer la baisse des effectifs liée à la pêche.[réf. souhaitée] Toutefois, les dernières données recueillies[réf. souhaitée] sur cet animal indiquent que le nombre de Requins soyeux est en baisse partout dans le monde, ce qui a incité l'Union internationale pour la conservation de la nature (UICN) à réévaluer son statut de conservation de « préoccupation mineure » à « quasi-menacé » en 2007.
Hiu sutra ((Inggris) silky shark) atau Carcharhinus falciformis adalah sebuah spesies hiu yang ditemukan di zona pelagik dan perairan dangkal di berbagai penjuru dunia. Hiu sutra adalah hiu terbesar dalam genis Carcharhinus, dengan ukuran mencapai 346 kilogram dan panjan 3.5 meter.[2] Kisaran panjang pejantan adalah sekitar 1.8 hingga 2.1 meter, sementara betina mempunyai kisaran sekitar 2.1 hingga 2.3 meter.[2] Secara umum hiu sutra hidup soliter, meskipun demikian, hiu ini telah diketahui dapat hidup secara berkelompok.[2] Mereka memangsa berbagai jenis ikan, cumi-cumi, kepiting.[2]
Meskipun terbiasa hidup di zona pelagik, hiu sutra juga dapat ditemukan di perairan dangkal.[3] Hiu ini telah dilaporkan terlihat pada perairan dengan kedalaman 18 meter.[3] Hiu sutra memilih perairan hangat, dengan suhu sekitar 23 °C, sebagai tempat hidupnya.[3] Hiu sutra banyak ditemukan dekat ujung landas kontinen dan di dekat terumbu karang di mana terdapat banyak makanan.[3]
Umumnya, hiu sutra menjelajahi perairan dengan kedalaman diatas 500 meter, tetapi hiu ini pernah ditangkap pada perairan dengan kedalaman sekitar 4000 meter.[3] Jantan dan betina dapat berenang secara bersamaan, akan tetapi hiu cenderung memilih berkawan dengan sesama hiu yang ukurannya yang serupa.[3] Biasanya, hiu yang lebih kecil dapat ditemukan di daerah pesisir, tempat di mana mereka membesarkan diri (daerah nursery), sedangkan hiu yang telah dewasa ditemukan di tengah laut dan perairan dalam.[3] Hiu sutra berukuran kecil biasanya ditemukan berdekatan dengan gerombolan ikan tuna.[3]
Hiu sutra adalah hiu terbesar dalam genis Carcharhinus, dengan ukuran mencapai 346 kilogram dan panjan 3.5 meter.[2] Kisaran panjang pejantan adalah sekitar 1.8 hingga 2.1 meter, sementara betina mempunyai kisaran sekitar 2.1 hingga 2.3 meter.[2] Hiu sutra dikenal dari moncongnya yang lebih pendek dari lebar mulutnya, daerah bubungan interdorsal (interdorsal ridge), sirip dorsal (sirip punggung) yang terletak di belakang sirip pektoral (sirip samping), dan sirip dorsal kedua yang terletak dekat sirip ekor.[4][5] Gigi hiu ini berbentuk segitiga dan gigi yang berada di dekat sudut mulut hiu memiliki kemiringan yang lebih besar.[4] Gigi hiu ini bergerigi, semakin dekat dengan ujung gigi, intensitas kerapatan gerigi tersebut semakin bertambah.[4] Warna hiu ini adalah perunggu coklat dan keputihan.[4] Ketika hiu mati, warna kilap pada kulit akan memudar dan warna kulit tersebut akan berubah menjadi abu-abu gelap.[4]
Hiu sutra jantan mampu mengeluarkan feromon, meskipun demikian, belum diketahui apakah feromon tersebut berperan dalam menarik pasanngan, mengusir pesaing, menandai wilayah, atau kombinasi dari ketiganya.[2] Ritual kawin dari hiu sutra, jikapun ada, masih belum diketahui secara pasti.[2] Jantan dapat kawin dengan beberapa betina saat musim kawin tiba.[2] Hiu sutra di perairan tropis tidak memiliki musim kawin yang tetap, jantan dan betina dapat melakukan perkawinan sepanjang tahun Sementara itu, hiu sutra di Teluk Meksiko mempunyai periode perkembang-biakan yang tetap, yaitu pada bulan-bulan musim panas (Juni, Juli, dan Agustus).[2] Hiu sutra yang baru lahir hidup mandiri dan menunjukkan kemampuan yang memamdai dalam berburu.[2]
Kematangan seksual pada betina dicapai oleh hiu sutra ketika menyentuh usia 7 hingga 9 tahun, dengan panjang tubuh 2,1 hingga 2,3 meter.[2] Pada jantan, kematangan tersebut dicapai pada usia 6 hingga 7 tahun, dengan panjang tubuh 1.8 hingga 2.1 meter.[2] Hiu sutra di Samudera India dan di Samudera Pasifik mencapai kematangan seksual pada usia yang lebih muda, dan pada ukuran yang lebih kecil dibandingkan dengan hiu yang tinggal di daerah lain (kira-kira 2 tahun lebih muda dan 0.6 meter lebih pendek).[2] Diperkirakan variasi kematangan seksual ini berhubungan dengan lingkungan hidup.[2] Pada perairan tropis yang lebih hangat, metabolisme dapat berjalan lebih cepat, sehingga laju pertumbuhan meningkat.[2] Meskipun demikian, hipotesis ini masih perlu dibuktikan secara empiris.[2]
Secara umum hiu sutra hidup soliter, meskipun demikian, hiu ini telah diketahui dapat hidup secara berkelompok.[2] Anakan pada umumnya bergerak dalam kelompok hingga mencapai kedewaasaan.[2] Strategi ini diperkirakan bertujuan untuk melindungi diri mereka dari predator yang lebih besar.[2] Hiu sutra dewasa dapat menunjukkan sifat sosial yang tinggi, dan dalam kelompoknya, dapat bercampur dengan hiu kepala martil (Sphyrna lewini).[2] Hewan ini aktif pada siang dan malam hari, akan tetapi puncak aktivitas hiu ini terjadi di waktu subuh.[2]
Hiu sutra merupakan spesies yang hidup bermigrasi, mengikuti pergerakan ikan berkelompok, seperti ikan tuna.[2] Mereka berpergian sendirian atau berkelompok.[2] Hiu sutra juga diketahui mempunyai perilaku yang agresif, dan pernah dilihat melakukan pertunjukkan intimidasi dengan meninggikan kepala, melekukkan punggung, dan merendahkan ekor.[2] Beberapa spesimen menunjukkan perilakuk ini untuk melindungi wilayah mereka dari pesaing dan predator.[2] Meskipun demikian, spesies ini memiliki rasa keingintahuan yang tinggi, dan menunjukkan keinginan untuk berinteraksi secara non-agresif dengan penyelam.[2]
Hiu sutra mempunyai mangsa yang terdiversifikasi. Mereka memangsa berbagai jenis ikan, cumi-cumi, kepiting.[2] Hiu sutra muda umumnya memangsa cumi-cumi, sementara yang dewasa lebih sering memangsa kepiting dan ikan kembung.[2] Di samping itu, ikan tuna sirip kuning (Diodon hystrix) pernah ditemukan dalam isi perut hiu sutra.[2]
Hiu sutra ((Inggris) silky shark) atau Carcharhinus falciformis adalah sebuah spesies hiu yang ditemukan di zona pelagik dan perairan dangkal di berbagai penjuru dunia. Hiu sutra adalah hiu terbesar dalam genis Carcharhinus, dengan ukuran mencapai 346 kilogram dan panjan 3.5 meter. Kisaran panjang pejantan adalah sekitar 1.8 hingga 2.1 meter, sementara betina mempunyai kisaran sekitar 2.1 hingga 2.3 meter. Secara umum hiu sutra hidup soliter, meskipun demikian, hiu ini telah diketahui dapat hidup secara berkelompok. Mereka memangsa berbagai jenis ikan, cumi-cumi, kepiting.
Lo squalo seta (Carcharhinus falciformis Müller & Henle, 1839), vedi: (Regolamento (UE) 2019/124 del Consiglio del 30 gennaio 2019 31.1.2019 L 29/1 G.U. dell'Unione Europea) e (G.U. della Repubblica Italiana 2ª Serie speciale - n. 59 del 01-08-2019), è uno squalo pelagico, conosciuto anche con il nome di squalo sericeo, di grandi dimensioni appartenente al genere Carcharhinus ed alla famiglia Carcharhinidae.
Si trovano in tutto il mondo, in mari a temperatura maggiore di 23 °C[1]. Di solito vive al largo, ma si può avvicinare alle coste, specialmente in prossimità di isole oceaniche isolate[1].
Nell'Oceano Atlantico, se ne trovano dallo stato del Massachusetts alla Spagna a nord, e dal Brasile meridionale all'Angola settentrionale a sud. Sono presenti nel Mar Mediterraneo, nel Golfo del Messico, nel Mar dei Caraibi. Abitano inoltre l'intero Oceano Indiano, tanto a sud da raggiungere il Mozambico da un lato e l'Australia occidentale dall'altro. Il Mar Rosso ed il Golfo Persico appartengono anch'essi all'areale. Nell'Oceano Pacifico, a nord l'areale raggiunge Cina e Giappone da un lato, la Baja California ed il Golfo di California dall'altro, a sud si estende dalla zona di Sydney, Australia alla Nuova Zelanda sino al Cile[1][2]. Basandosi su dati storici, quattro differenti popolazioni sono state individuate: atlantica nordoccidentale, pacifica centroccidentale, pacifica orientale e indiana[2].
Vivono principalmente in oceano aperto, e sono più comunemente avvistati nella zona compresa tra la superficie fino alla profondità di 200 metri. Possono spingersi fino a 500 metri di profondità[1], che superano difficilmente[3], anche se un esemplare è stato avvistato addirittura a 4000[4].
Studi specifici compuiti nell'area tropicale pacifica e nel Golfo del Messico nordoccidentale hanno provato che questi squali spendono il 99% del loro tempo al di sopra dei 50 metri di profondità, e l'80-85% del tempo in acque tra i 26 ed i 30 °C; tutto questo indipendentemente dall'ora del giorno o della notte[5][6].
La specie predilige i bordi delle piattaforme continentali ed insulari, al di sopra di barriere coralline profonde e presso isole. Sulle coste dei continenti l'areale si estende molto più a nord ed a sud che in oceano aperto. Può avventurarsi in acque costiere profonde solo 18 metri.[7].
Si muovono molto e migrano, anche se i dettagli del processo di migrazione sono poco noti. Alcuni esemplari sono stati osservati mentre si spostavano anche di 60 km al giorno e coprivano distanze totali fino a 1339 km[8]. Gli esemplari di grandi dimensioni si spostano in genere di più. Nel Pacifico, ma probabilmente anche altrove, spendono i mesi invernali a latitudini più grandi, in modo particolare durante gli anni in cui l'effetto di El Niño è più consistente[9][10]. Nell'Atlantico settentrionale, la maggior parte di questi squali segue la Corrente del Golfo a nord lungo la East Coast[8]. Nel Golfo di Aden, è più comune incontrarli in tarda primavera ed estate[2].
La forma del corpo è quella tipica degli squali nell'immaginario collettivo, snella e con la pelle liscia e setosa. Presenta un muso arrotondato, gli occhi grandi e la bocca relativamente piccola[11]. I denti presentano cuspidi[11].Sul dorso il colore è marrone grigiastro e sul ventre è bianco senza tessiture particolari[11]. Ciò che distingue il Carcharhinus falciformis dagli altri squali è la seconda pinna dorsale, dotata di una punta che si eleva per un'altezza pari a 2 volte e mezza quella della pinna stessa[11]. La prima dorsale è inoltre posta molto più indietro di quella degli altri Carcharhiniformes: la sua base è all'indietro rispetto alle pinne pettorali[12][13]. La lunghezza massima registrata è di 3.5 metri[14], ma generalmente non superano i 2.4 metri.
Il Carcharhinus falciformis è più attivo, anche se meno aggressivo, degli altri due squali pelagici, la verdesca (Prionace glauca) e lo squalo longimanus (Carcharhinus longimanus). Si avvicina alle coste più frequentemente delle altre due specie, ma rimane uno squalo pelagico, anche perché le sue sortite vicino a terra rimangono rare in termini assoluti. Molto più facilmente lo si trova vicino a barriere coralline con pendenze elevate. Si tratta di un pesce solitario[15], anche se spesso è accompagnato da banchi di tonni[1].
Sono predatori opportunisti, che si nutrono principalmente di pesci ossei sia al largo che vicino alle coste, ma anche di calamari, granchi ed argonauta[16]. Vi sono evidenze fossili di sciacallaggio su carcasse di balena
Tra i pesci che cadono preda di questi squali troviamo tonni, sgombri, sardine, cernie, pesci delle famiglie Mugilidae e Lutjanidae e del genere Decapterus, chifosidi, pesci gatto di mare, anguille, pesci lanterna, monacantidi, pesci balestra, pesci istrice[1][2][17].
In presenza di grandi quantità di cibo si riuniscono in gruppi numerosi. Uno di questi gruppi è stato osservato nel Pacifico mentre costringeva pesci più piccoli a stringersi uno addosso all'altro in una massa compatta vicino alla superficie, in modo da intrappolarli e poterli divorare più facilmente[2]. In questi casi gli squali non fanno altro che attraversare i banchi di prede con la fauci spalancate e li bloccano ai lati della mascella. Anche se la caccia avviene in gruppo, ogni individuo porta gli attacchi singolarmente ed indipendentemente dagli altri[18].
Alcuni studi condotti al largo della Florida e delle Bahamas hanno evidenziatò la forte sensibilità della specie ai suoni, in particolare agli impulsi irregolari a bassa frequenza (nella banda 10-20 Hz). In vari esperimenti, questi suoni riprodotti sott'acqua hanno attirato squali lontani centinaia di metri. Gli squali in questione si orientano in base a questi suoni perché sono simili a quelli emessi da prede quali uccelli o delfini, e pertanto indicano fonti di cibo promettenti[18][19]. Gli stessi studi hanno dimostrato come dopo essere stato attirato dal suono, uno di questi squali si ritira rapidamente se il suono cambia improvvisamente in ampiezza o tono. La reazione non è necessariamente legata alla comparsa di predatori che emettono suoni di disturbo. Se esposti ripetutamente a questi cambiamenti di tonalità ed ampiezza, i Carcharhinus falciformis vi si abituano e smettono di fuggire, anche se l'apprendimento richiede più tempo che nel più coraggioso Carcharhinus longimanus[20].
La forza del morso delle mascelle lunghe 2 m è stata misurata e raggiunge gli 890 N[21]. Vi è un'interessante relazione tra questa specie ed i tonni: al largo del Ghana, praticamente ogni banco di tonni ha uno di questi squali che la insegue, e nel Pacifico orientale i danni agli attrezzi da pesca sono così ingenti che i pescatori di tonni hanno soprannominato la specie squalo mangiatore di reti[1][22]. Questi squali rivaleggiano con i delfini dal naso a bottiglia per il cibo, quando entrambi gli animali prendono di mira lo stesso banco di prede. La quantità di cibo che i delfini riescono a catturare si riduce in presenza di squali. se gli squali sono in grande numero, tendono ad occupare il centro del banco, mentre i delfini sono confinati alla periferia, probabilmente in maniera volontaria, in modo da evitare di rimanere feriti durante gli attacchi dei pescecani. Al contrario, se i delfini si riuniscono in numero sufficiente, sono in grado di allontanare gli squali. Indipendentemente da chi dei due animali sia in vantaggio, squali e delfini non hanno attaggiamenti esageratamente aggressivi verso l'un l'altro[23].
Come le altre specie della famiglia, questi squali sono vivipari e gli embrioni, una volta esaurita la riserva di tuorlo si nutrono durante la crescita attraverso il sacco vitellino, dopo che questi si è trasformato in una connessione placentale tra figlio e madre. Contrariamente a quanto accade per altri squali vivipari, la placenta di questa specie è diversa da quella dei mammiferi per il fatto che non vi è mescolanza di tessuti tra madre e figlio. Inoltre, i globuli rossi fetali sono molto più piccoli di quelli materni, al contrario di ciò che avviene nei mammiferi. Le femmine adulte hanno un unico ovario funzionale (sul lato destro) e due uteri funzionali, che sono divisi longitudinalmente in compartimenti da dedicare ai diversi embrioni[24].
Si ritiene che quasi ovunque si riproducano per tutto l'anno, mentre l'accoppiamento nel Golfo del Messico avviene in tarda primavera o all'inizio dell'estate (da maggio ad agosto)[7][25]. Errori nella raccolta dati possono comunque aver oscurato comportamenti stagionali anche in altre zone[2]. Le femmine mettono al mondo la prole ogni anno o ogni due dopo una gestazione di 12 mesi circa[26]. Le dimensioni della cucciolata possono essere comprese tra 2 e 14[16], ma altre fonti individuano i limiti in 1 e 16 esemplari, il numero essendo più grande in relazione alle dimensioni della madre. Il numero tipico è di 6-12 squaletti[2].
La nascita avviene in apposite aree nido sul bordo esterno della piattaforma continentale, dove molto cibo è disponibile e attacchi da parte di grossi squali di altre specie che vivono al largo sono meno probabili. Il rischio legato alla predazione ha sviluppato nei cuccioli una capacità di crescita molto rapida, che gli permette di crescere di 25–30 cm nel primo anno di vita. Dopo qualche mese (o prima del primo inverno nel Golfo del Messico) il giovane squalo abbandona l'area protetta per inoltrarsi in oceano aperto[2][18][25].
La vita e la crescita di questi squali varia all'interno dell'areale (vedi tabella). Gli esemplari dell'Atlantico nordoccidentale sono tendenzialmente più grandi di quelli del Pacifico centro occidentale a tutte le età, mentre gli individui del Pacifico orientale sono più piccoli di tutti gli altri. Gli esemplari atlantici orientali ed indiani sembrano raggiungere e superare la lunghezza degli atlantici nordoccidentali, ma non vi sono dati a sufficienza per esserne certi[2].
Il tasso di crescita è in generale moderato se confrontato con squali di altre specie e simile per entrambi i sessi, anche se può variare sensibilmente tra i singoli esemplari. Nel Pacifico centrale ad esempio, uno studio ha asserito una crescita più lenta delle femmine rispetto ai maschi, ma può darsi che pochi dati su femmine di grandi dimensioni fossero disponibili[7]. La crescita rapida è stata osservata nel Golfo del Messico settentrionale, la più lenta presso la costa nordorientale di Taiwan[32]. Maschi e femmine raggiungono la maturità sessuale all'età di 6-10 anni e 7-12 anni rispettivamente[2]. Gli squali che vivono in acque temperate crescono più lentamente di quelli in acque calde[32]. La vita massima è di almeno 22 anni[27].
Per i sub non costituisce generalmente un grande pericolo, ma in presenza di pesci fiocinati o se approcciato in modo troppo diretto può diventare aggressivo e di conseguenza è considerato potenzialmente pericoloso.
Lo squalo seta (Carcharhinus falciformis Müller & Henle, 1839), vedi: (Regolamento (UE) 2019/124 del Consiglio del 30 gennaio 2019 31.1.2019 L 29/1 G.U. dell'Unione Europea) e (G.U. della Repubblica Italiana 2ª Serie speciale - n. 59 del 01-08-2019), è uno squalo pelagico, conosciuto anche con il nome di squalo sericeo, di grandi dimensioni appartenente al genere Carcharhinus ed alla famiglia Carcharhinidae.
De zijdehaai[3] (Carcharhinus falciformis), ook wel kanhaai,[4] is een haai uit de familie van de requiemhaaien.[5][6]
De zijdehaai komt wereldwijd voor in de tropische wateren. In meer detail:
De zijdehaai (Carcharhinus falciformis), ook wel kanhaai, is een haai uit de familie van de requiemhaaien.
Żarłacz jedwabisty[3] (Carcharhinus falciformis) – gatunek ryby chrzęstnoszkieletowej z rodziny żarłaczowatych (Carcharhinidae), rekin drapieżny. Był też opisywany pod nazwą żarłacz Menisora[4] (Carcharias menisorrah).
Spotykany w większości ciepłych (od tropikalnych do umiarkowanych) wód oceanicznych. Głównie pelagial, ale czasami podchodzi do brzegu w pobliżu wysp. Aksamitna gładka skóra. Osiąga długość do zazwyczaj 2,4 metrów (maksymalnie 3,5 m). Jest szybkim i bardzo zwinnie pływającym drapieżnikiem. Znajduje się na światowej liście gatunków zagrożonych.
Żarłacz jedwabisty (Carcharhinus falciformis) – gatunek ryby chrzęstnoszkieletowej z rodziny żarłaczowatych (Carcharhinidae), rekin drapieżny. Był też opisywany pod nazwą żarłacz Menisora (Carcharias menisorrah).
O tubarão-seda (Carcharhinus falciformis), também conhecido por inúmeros nomes como tubarão-lombo-preto, tubarão-baleia-cinza, tubarão-oliva, tubarão-falciforme, e tubarão-foice, é uma espécie de tubarão requiem da família Carcharhinidae, nomeado pela textura suave de sua pele. É um dos tubarões mais abundantes na zona pelágica e pode ser encontrado em todo o mundo em águas tropicais. Altamente móvel e migratório, esse tubarão é encontrado com mais freqüência na borda da plataforma continental até 50 m (164 pés). O tubarão-seda tem um corpo esbelto e aerodinâmico e normalmente cresce até um comprimento de 2,5 m (8 pés 2 pol). Ele pode ser diferenciado de outros grandes tubarões requiem por sua primeira barbatana dorsal relativamente pequena com uma margem traseira curvada, sua minúscula segunda barbatana dorsal com uma ponta traseira longa e livre e suas longas barbatanas peitorais em forma de foice. É um profundo, metálico cinza-bronze acima e branco abaixo.
Com presas muitas vezes escassas em seu ambiente oceânico, o tubarão-seda é um caçador rápido, curioso e persistente. Alimenta-se principalmente de peixes ósseos e cefalópodes e é conhecido por levá-los a cardumes compactados antes de lançar ataques de boca aberta e cortante. Esta espécie geralmente arrasta cardumes de atum, uma de suas presas favoritas. Seu senso auditivo é extremamente agudo, permitindo localizar os ruídos de baixa frequência gerados por outros animais alimentadores e, por extensão, fontes de alimento. O tubarão-seda é vivíparo, o que significa que os embriões em desenvolvimento são sustentados por uma conexão placentária com a mãe. Variação geográfica significativa é vista em seus detalhes do ciclo de vida. A reprodução ocorre o ano todo, exceto no Golfo do México, onde segue um ciclo sazonal. As fêmeas dão à luz ninhadas de até 16 filhotes anualmente ou bienalmente. Os tubarões recém-nascidos passam seus primeiros meses em viveiros de recifes relativamente abrigados na plataforma continental externa, crescendo substancialmente antes de se mudarem para o oceano aberto.
O tamanho grande e os dentes cortantes do tubarão-seda o tornam potencialmente perigoso e se comporta agressivamente em relação aos mergulhadores. No entanto, os ataques são raros, pois poucos humanos entram em seu habitat oceânico. Os tubarões-seda são valorizados por suas barbatanas e, em menor grau, por sua carne, pele, óleo de fígado e mandíbulas. Devido à sua abundância, eles formam um componente importante da pesca comercial e artesanal de tubarões em muitos países. Além disso, sua associação com o atum resulta em muitos tubarões sendo capturados como capturas acessórias na pesca de atum. Embora com reprodução lenta, como a maioria dos outros tubarões, se pensava que a ampla distribuição e o grande tamanho populacional do tubarão lombo-preto protegiam as espécies contra essas pressões de pesca. No entanto, os dados agora sugerem que o número de tubarões-seda está diminuindo em todo o mundo, o que levou a IUCN a reavaliar seu status de conservação para Vulnerável em 2017.
Uma descrição científica do tubarão-seda foi publicada pela primeira vez pelos biólogos alemães Johannes Müller e Jakob Henle, sob o nome Carcharias (Prionodon) falciformis, em seu Systematische Beschreibung der Plagiostomen, de 1839. Os autores subsequentes atribuíram essa espécie ao gênero Carcharhinus.[4][5] Como o espécime do tipo Müller e Henle era um feto feminino de 53 cm de comprimento em Cuba, historicamente os tubarões da seda não eram reconhecidos como C. falciformis e foram descritos como uma espécie separada, Carcharhinus floridanus, de Henry Bigelow, William Schroeder e Stewart Springer, em 1943. Jack Garrick, Richard Backus e Robert Gibbs, Jr. sinonimizaram C. floridanus com C. falciformis em 1964.[6]
O epíteto específico falciformis é em latim para "foice", que se refere ao contorno das barbatanas dorsal e peitoral.[2] O nome comum do tubarão-seda vem da textura fina de sua pele em comparação com outros tubarões, um produto de seus minúsculos dentículos dérmicos densamente compactados.[7] Também pode ser chamado de tubarão lombo-preto (geralmente usado para C. sealei), tubarão-cinzento (geralmente usado para C. amblyrhynchos), tubarão-baleia cinza, tubarão-oliva, tubarão-de-recife, tubarão ridgeback, tubarão falciforme, tubarão em forma de foice, tubarão da seda e baleeiro sedoso.[8][3]
Carcharhinus falciformis
Dentes fossilizados pertencentes ao tubarão-seda foram encontrados na Carolina do Norte: nas proximidades de duas baleias, uma na lama que data do Pleistoceno-Holoceno (cerca de 12.000 anos atrás) e a outra na pedra calcária de Goose Creek que data do Plioceno tardio ( cerca de 3,5 milhões de anos atrás ), bem como do rio Pungo, datando do Mioceno (23-5,3 milhões de anos atrás ).[10][11] Dentes fósseis também foram encontrados nos estratos do Plioceno na pedreira de Cava Serredi, em Toscana, Itália.[12] Carcharhinus elongatus, um representante anterior de sua linhagem com dentes de bordas lisas, é conhecido dos depósitos de oligoceno (34–23 milhões de anos atrás ) na formação de Igreja Velha da Virgínia e na formação Ashley da Carolina do Sul. Um conjunto de dentes Eoceno (56-34 milhões de anos atrás ) mal descritos, semelhantes aos desta espécie, são conhecidos do Egito.[11]
Os esforços iniciais para resolver as relações evolutivas do tubarão-seda foram inconclusivos; com base na morfologia, Jack Garrick, em 1982, sugeriu o tubarão da mancha preta (C. sealei) como seu parente mais próximo.[13] Em 1988, Leonard Compagno o atribuiu feneticamente a um "grupo de transição" informal, também contendo o tubarão de nariz preto (C. acronotus), o tubarão de pontas negras (C. melanopterus), o tubarão nervoso (C. cautus), o tubarão de cobre (C. brachyurus) e o tubarão noturno (C. signatus).[14]
Mais recentemente, a análise filogenética de Gavin Naylor, de 1992, baseada em dados da sequência de aloenzimas, descobriu que o tubarão-seda faz parte de um grupo contendo grandes tubarões com uma crista entre as barbatanas dorsais. Um ramo dentro deste grupo contém o tubarão-corre-costa (C. plumbeus) e o tubarão de focinho longo (C. altimus), enquanto o tubarão-seda é o membro basal do outro ramo e o táxi irmão de um clado contendo o tubarão de recife do Caribe (C. perezi), tubarão de Galápagos (C. galapagensis), tubarão oceânico (C. longimanus), tubarão sombrio (C. obscurus) e tubarão azul (Prionace glauca).[9] A análise do DNA ribossômico de 2008 de Mine Dosay-Abkulut, que incluiu os tubarões-seda, azul e de focinho longo, confirmou a proximidade dessas três espécies.[15]
O tubarão-seda tem uma distribuição cosmopolita em águas marinhas mais quentes que 23 ° C (73 ° F). No Oceano Atlântico, é encontrado do estado americano de Massachusetts a na Espanha, no norte, e do sul do Brasil ao norte de Angola, no sul, incluindo o Mar Mediterrâneo, Golfo do México e Mar do Caribe. Ocorre em todo o Oceano Índico, até o sul de Moçambique no oeste e na Austrália Ocidental no leste, incluindo o Mar Vermelho e o Golfo Pérsico. No Oceano Pacífico, a extensão norte de seu alcance vai do sul da China e do Japão ao sul da Baja Califórnia e no Golfo da Califórnia, enquanto a extensão sul vai de Sydney, na Austrália, ao norte da Nova Zelândia e ao norte do Chile.[2][4] Com base nas diferenças do ciclo de vida, quatro populações distintas de tubarões-seda foram identificadas em bacias oceânicas em todo o mundo: no Atlântico noroeste, no Pacífico oeste e central, no Pacífico oriental e no Oceano Índico.[2]
Principalmente um habitante do oceano aberto, o tubarão-seda é mais comum da superfície até uma profundidade de 200 m (660 pés), mas pode mergulhar até 500 m (1.600 pés) ou mais.[4] Estudos de rastreamento no Pacífico tropical oriental e no norte do Golfo do México descobriram que os tubarões-seda passam 99% de seu tempo a 50 m (160 pés) da superfície e 80 a 85% de seu tempo na água a uma temperatura de 26 – 30 ° C (79 – 86 ° F); o padrão era constante, independentemente do dia ou da noite.[16][17] Esta espécie favorece as bordas das plataformas continentais e insulares, geralmente sobre recifes de águas profundas e ao redor das ilhas. Seu alcance se estende mais ao norte e sul ao longo das margens continentais do que nas águas oceânicas. Ocasionalmente, pode se aventurar em águas costeiras tão rasas quanto 18 m (59 pés).[18] Os tubarões-da-seda são altamente móveis e migratórios, embora os detalhes de seus movimentos sejam pouco conhecidos. Os dados de marcação registraram tubarões individuais movendo-se até 60 km (37 milhas) por dia e cobrindo distâncias de até 1.339 km (832 milhas).[19] Os tubarões maiores geralmente se movem distâncias maiores que os menores. No Oceano Pacífico e possivelmente em outros lugares, ele passa o verão em latitudes ligeiramente mais altas, principalmente durante os anos mais quentes do El Niño.[20][21] No norte do Atlântico, a maioria dos tubarões segue a corrente do Golfo ao norte, ao longo da costa leste dos EUA.[19] No Golfo de Áden, é mais comum no final da primavera e no verão.[2]
Esbelto e aerodinâmico, o tubarão-seda tem um focinho bastante longo e arredondado, com abas de pele pouco desenvolvidas na frente das narinas. Os olhos circulares de tamanho médio são equipados com membranas nictitantes (terceiras pálpebras protetoras). Sulcos curtos e rasos estão presentes nos cantos da boca.[4][22] 14 a 16 e 13 a 17 fileiras de dentes são encontradas em ambos os lados das mandíbulas superior e inferior, respectivamente (normalmente 15 para ambas). Os dentes superiores são triangulares e fortemente serrilhados, com um entalhe na borda posterior; eles estão eretos no centro e tornam-se mais oblíquos para os lados. Os dentes inferiores são estreitos, eretos e com bordas lisas. Os cinco pares de fendas branquiais são de comprimento moderado.[23]
As barbatanas dorsal e peitoral são distintas e ajudam a distinguir o tubarão-seda de espécies semelhantes. A primeira barbatana dorsal é relativamente pequena, medindo menos de um décimo da altura do tubarão, e se origina atrás das pontas traseiras livres das barbatanas peitorais. Possui um ápice arredondado, uma margem traseira em forma de "S" e uma ponta traseira livre com cerca da metade do comprimento da barbatana. A segunda barbatana dorsal é minúscula, menor que a anal, com uma ponta traseira livre prolongada até três vezes enquanto a barbatana for alta. Uma crista dorsal estreita corre entre as barbatanas dorsais. As barbatanas peitorais são estreitas e em forma de foice, e particularmente longas em adultos. A barbatana anal se origina um pouco à frente da segunda barbatana dorsal e possui um entalhe profundo na margem posterior. A barbatana caudal é bastante alta com um lobo inferior bem desenvolvido.[4][22]
A pele é densamente coberta por minúsculos dentículos dérmicos sobrepostos. Cada dentículo dérmico é em forma de diamante e possui sulcos horizontais levando a dentes marginais posteriores, que aumentam em número à medida que o tubarão cresce.[6][7] O dorso é marrom dourado metálico a cinza escuro e a barriga é branca como a neve, que se estende até o flanco como uma faixa mais clara e leve. As barbatanas (exceto a primeira dorsal) escurecem nas pontas; isso é mais óbvio em jovens tubarões.[4][7] A coloração desaparece rapidamente para um cinza opaco após a morte.[24] Um dos maiores membros de seu gênero, o tubarão-seda geralmente atinge um comprimento de 2,5 m (8,2 pés), com um comprimento e peso máximo registrados de 3,5 m (11 pés) e 346 kg (763 lb), respectivamente.[8] As fêmeas crescem mais que os machos.[7]
O tubarão-seda é um dos três tubarões pelágicos mais comuns, juntamente com os tubarões-azul e oceânicos, e conta entre os mais numerosos grandes animais oceânicos do mundo, com uma população de pelo menos dezenas de milhões.[25] Comparado às outras duas espécies, é menos estritamente pelágico com o maior número encontrado em águas marinhas associadas à terra, onde os alimentos são mais facilmente obtidos do que em alto mar. O tubarão-seda é um predador ativo, curioso e agressivo, embora adie ao tubarão oceânico mais lento, porém mais poderoso, em situações competitivas.[4] Ao abordar algo de interesse, pode parecer desatento, circulando tranquilamente e às vezes balançando a cabeça de um lado para o outro. No entanto, ele pode responder com rapidez surpreendente a qualquer mudança em seu entorno imediato.[26] Esse tubarão geralmente é encontrado em torno de objetos flutuantes, como toras ou bóias navais amarradas.[27]
Sabe-se que tubarões-seda mais jovens formam agregações grandes e pouco organizadas, possivelmente para defesa mútua.[28] Durante as migrações, mais de mil indivíduos podem se reunir.[29] Esses grupos geralmente são segregados por tamanho e no Pacífico, talvez também por sexo.[7][20][30] Observou-se que os tubarões-seda de um grupo "inclinam-se", apresentando todo o perfil lateral um em direção ao outro, bem como abrem as mandíbulas ou sopram as brânquias. Na ocasião, tubarões também foram vistos subitamente subindo, se afastando pouco antes de chegar à superfície e deslizando de volta para águas mais profundas. O significado desses comportamentos é desconhecido.[26] Quando confrontado, o tubarão-seda pode apresentar uma ameaça, na qual arqueia as costas, abaixa a cauda e as barbatanas peitorais e eleva a cabeça. O tubarão então começa a nadar em curvas apertadas, com um movimento rígido e irregular, muitas vezes virando o rumo da ameaça percebida.[31]
Os predadores em potencial do tubarão-seda incluem tubarões maiores e baleias assassinas (Orcinus orca).[32] Os parasitas conhecidos deste tubarão incluem o isópode Gnathia trimaculata,[33] o copépode Kroeyerina cortezensis,[34] e as tênias Dasyrhynchus variouncinatus e Phyllobothrium sp.[35][36] Tubarões-seda freqüentemente se misturam com cardumes de tubarões-martelo-recortado (Sphyrna lewini) e são conhecidos por seguir mamíferos marinhos. Um relato do Mar Vermelho descreve 25 tubarões-seda perseguindo uma grande vagem de golfinhos (Tursiops sp.), Juntamente com 25 tubarões cinzentos de corais (C. amblyrhynchos) e um tubarão solitário (C. albimarginatus). Os próprios tubarões seda são acompanhados por peixes-piloto juvenis (Naucrates ductor), que "cavalgam" a onda de pressão à frente do tubarão, bem como por Carangidae, que arrancam restos de comida e esfregam a pele do tubarão para eliminar os parasitas.[28][37]
O tubarão-seda é um predador oportunista, alimentando-se principalmente de peixes ósseos de todos os níveis da coluna de água, incluindo atum, cavala, sardinha, salmonete, garoupas, Lutjanidae, carpas, Kyphosidae, chubs marinhos, bagres do mar, enguias, peixes-lanterna, peixes-arquivo, peixes-gatilho e peixes-porco. Também pode ]alimentar-se de lulas, nautilus de papel e caranguejos, e evidências fósseis indicam que ele foi varrido nas carcaças de baleias.[2][4][10] Boas oportunidades de alimentação podem atrair tubarões-seda em grande número; um desses agregados de alimentação no Pacífico foi documentado "pastoreando" um cardume de peixes pequenos em uma massa compacta (uma bola de isca) e prendendo-o contra a superfície, após o que os tubarões consumiram todo o cardume.[2] Ao atacar peixes bem compactados, os tubarões-baleia cinzentos atacam a massa e cortam a boca aberta, pegando as presas nos cantos de suas mandíbulas. Embora vários indivíduos possam se alimentar de uma só vez, cada um lança seu ataque de forma independente.[28]
Estudos realizados na costa da Flórida e nas Bahamas mostraram que tubarões-seda são altamente sensíveis ao som, em particular pulsos irregulares de baixa frequência (10 a 20 Hz). Experimentos em que esses sons eram tocados debaixo d'água atraíram tubarões a centenas de metros de distância. Os tubarões seda provavelmente se orientam a esses sons porque são semelhantes ao ruído gerado pela alimentação de animais como pássaros ou golfinhos, indicando assim fontes promissoras de alimento.[26][28] Esses estudos também demonstraram que um tubarão seda atraído por um som se retrai rapidamente se esse som mudar abruptamente em amplitude ou caráter; essa mudança não precisa ser um som produzido por um predador para evocar a reação. Em exposições repetidas, os tubarões-seda habituam-se à mudança de som e param de se retirar, embora demorem muito mais para fazer isso em comparação com o tubarão oceânico, mais ousado.[32]
A força de mordida de um tubarão-seda de 2 m de comprimento foi medida em 890 newtons (200 lbf).[38] Existe uma associação bem estabelecida entre essa espécie e o atum: em Gana, quase todos os cardumes de atum têm tubarões-seda e, no leste do Pacífico, esses tubarões causam tais danos às artes de pesca do atum e às capturas que os trabalhadores da pesca lhes deram o apelido "tubarões devoradores de redes".[4][24] Tubarões-seda e golfinhos-nariz-de-garrafa competem quando ambas as espécies têm como alvo o mesmo cardume de peixes; a quantidade consumida pelos golfinhos diminui em relação ao número de tubarões presentes. Se um grande número de tubarões estiver presente, eles tendem a permanecer dentro do cardume de presas, enquanto os golfinhos se consignam à periferia, possivelmente para evitar ferimentos acidentais causados pelos ataques cortantes dos tubarões. Por outro lado, se um grupo grande o suficiente de golfinhos se reunir, eles poderão afugentar os tubarões do cardume de presas. Independentemente de qual deles domine, os dois predadores não se envolvem em nenhum comportamento abertamente agressivo um contra o outro.[39]
Como outros membros de sua família, o tubarão-seda é vivíparo: uma vez que o embrião em desenvolvimento esgota seu suprimento de gema, o saco de gema esgotado é convertido em uma conexão placentária através da qual a mãe fornece alimento. Em relação a outros tubarões vivíparos, a placenta do tubarão-seda é menos semelhante à estrutura análoga dos mamíferos, pois não existe interdigitação entre os tecidos do feto e da mãe. Além disso, as células vermelhas do sangue fetal são muito menores que as células maternas, o que é oposto ao padrão observado nos mamíferos. As fêmeas adultas têm um único ovário funcional (no lado direito) e dois úteros funcionais, que são divididos longitudinalmente em compartimentos separados para cada embrião.[40]
Pensa-se que os tubarões-seda em muitas partes do mundo se reproduzem o ano todo, enquanto o acasalamento e o nascimento no Golfo do México ocorrem no final da primavera ou no início do verão (maio a agosto).[18][30] No entanto, em alguns casos, a presença de sazonalidade reprodutiva pode ter sido obscurecida por vieses na coleta de dados.[2] As fêmeas dão à luz após um período de gestação de 12 meses, todos os anos ou a cada dois anos.[1] O tamanho da ninhada varia de 1 a 16 e aumenta com o tamanho da fêmea, sendo 6 a 12 típicos.[2] Os filhotes nascem em áreas de viveiro de recifes na plataforma continental externa, onde ocorrem amplos suprimentos alimentares e proteção contra grandes tubarões pelágicos. O risco de predação optou por um crescimento rápido em tubarões jovens, que crescem 25 a 30 cm de comprimento no primeiro ano de vida. Após alguns meses (ou no primeiro inverno no Golfo do México), os tubarões agora subadultos migram do berçário para o oceano aberto.[2][28][30]
As características do ciclo de vida do tubarão-seda diferem em sua faixa (consulte a tabela). Os tubarões do Noroeste do Atlântico tendem a ser maiores do que os do Pacífico Centro-Oeste em todas as idades, enquanto os tubarões do Pacífico Oriental tendem a ser menores do que os tubarões de outras regiões. Os tubarões do Atlântico Leste e do Oceano Índico parecem coincidir ou exceder o tamanho dos tubarões do noroeste do Atlântico, mas os números são baseados em relativamente poucos indivíduos e são necessários mais dados.[2]
A taxa geral de crescimento do tubarão-seda é moderada em comparação com outras espécies de tubarões e semelhante para ambos os sexos, embora varie significativamente entre os indivíduos. Um estudo na região central do Pacífico descobriu que as fêmeas crescem muito mais lentamente que os machos, mas os resultados podem ter sido distorcidos pela falta de dados de fêmeas grandes.[18] As taxas de crescimento mais altas relatadas são de tubarões no norte do Golfo do México e as mais baixas de tubarões do nordeste de Taiwan.[45] Machos e fêmeas atingem a maturidade sexual entre 6 e 10 anos e 7 a 12 anos ou mais, respectivamente.[2] Tubarões de águas mais temperadas podem crescer mais devagar e amadurecer mais tarde do que aqueles em regiões mais quentes.[45] A vida útil máxima é de pelo menos 22 anos.[25]
Dado seu tamanho formidável e dentição, o tubarão-seda é considerado potencialmente perigoso para os seres humanos. No entanto, raramente entra em contato com as pessoas devido a seus hábitos oceânicos.[7] Sua curiosidade e ousadia naturais podem levá-lo a abordar repetidamente e de perto os mergulhadores, e podem tornar-se perigosamente excitados na presença de alimentos. O tubarão-seda tende a ser mais agressivo se encontrado em um recife do que em águas abertas. Casos de tubarões individuais perseguindo persistentemente mergulhadores e até forçando-os a sair da água foram relatados.[37][47] Em maio de 2009, o International Shark Attack File lista seis ataques atribuíveis ao tubarão-seda, três deles não provocados e nenhum fatal.[48]
Um grande número de tubarões-seda é capturado pelas pescarias comerciais e artesanais de tubarões multiespécies que operam no México, Guatemala, El Salvador, Costa Rica, Estados Unidos, Equador, Espanha, Portugal, Sri Lanka, Maldivas, Iêmen e Costa do Marfim. Um número ainda maior é capturado incidentalmente pela pesca de palangre e redes de cerco com retenida em toda a sua extensão, particularmente as que utilizam dispositivos de agregação de peixes. É o tubarão mais comum capturado como captura acessória no leste do Pacífico e no Golfo do México, e o segundo tubarão mais comum capturado como captura acessória (ao lado do tubarão azul) em geral.[2][49] As barbatanas são valorizadas como um ingrediente na sopa de barbatana de tubarão, com os tubarões capturados frequentemente sofrendo Shark finning e jogados no mar e o resto do corpo descartado. As barbatanas de um milhão e meio de tubarões-seda estimados são comercializadas globalmente por ano; é a segunda ou terceira espécie mais comuns leiloadas no mercado de barbatanas de Hong Kong, o que representa mais da metade do comércio global.[1][2] A carne (vendida fresca ou seca e salgada), a pele e o óleo de fígado também podem ser utilizados,[4] e também os maxilares: essa espécie é a fonte predominante de mandíbulas secas de tubarão vendidas a turistas nos trópicos.[28] Alguns pescadores esportivos capturam tubarões-seda.[7]
Como um dos tubarões mais abundantes e amplamente distribuídos na Terra, o tubarão-seda já foi considerado imune ao esgotamento, apesar da forte mortalidade por pesca. Somente em 1989, cerca de 900.000 indivíduos foram capturados como capturas acessórias na pesca com palangre do atum do sul e central do Pacífico, aparentemente sem efeito sobre a população total.[25] Os dados da pesca neste tubarão são frequentemente confundidos com a subnotificação, falta de separação no nível das espécies e identificação problemática. No entanto, evidências crescentes indicam que o tubarão-seda declinou substancialmente em todo o mundo, uma conseqüência de sua modesta taxa reprodutiva que é incapaz de sustentar níveis tão altos de exploração. As capturas anuais totais relatadas à Organização para a Agricultura e a Alimentação caíram de 11.680 toneladas em 2000 para 4.358 toneladas em 2004. As avaliações regionais encontraram tendências semelhantes, estimando quedas de cerca de 90% no Pacífico central entre as décadas de 1950 e 1990, 60% na Costa Rica de 1991 a 2000, 91% no Golfo do México entre as décadas de 1950 e 1990 e 85% (para todos os grandes tubarões requiem) no noroeste do Atlântico entre 1986 e 2005. A pesca de tubarões sedosos no Sri Lanka relatou uma de uma captura de pico de 25.400 toneladas em 1994 para apenas 1.960 toneladas em 2006, indicativo de um colapso local do estoque. No entanto, as pescarias japonesas nos oceanos Pacífico e Índico não registraram mudanças na taxa de captura entre as décadas de 1970 e 1990,[1] e a validade das metodologias usadas para avaliar declínios no Golfo do México e no noroeste do Atlântico tem sofrido muito debate.[50][51][52]
A partir de 2017, o tubarão-seda é classificado pela União Internacional para Conservação da Natureza (IUCN) como uma espécie vulnerável. O tubarão-seda está listado no Anexo I, Espécies Altamente Migratórias, da Convenção das Nações Unidas sobre o Direito do Mar, embora isso ainda deva resultar em quaisquer esquemas de gestão. As espécies devem se beneficiar da proibição de barbatanas de tubarões, que estão sendo cada vez mais implementadas por nações e entidades supranacionais, incluindo os Estados Unidos, a Austrália e a União Européia.[1] Organizações como a Comissão Internacional para a Conservação do Atum Atlântico e a Comissão Interamericana de Atum Tropical também adotaram medidas para melhorar o monitoramento da pesca, com o objetivo final de reduzir as capturas acessórias de tubarões.[2] No entanto, dada a natureza altamente migratória do tubarão-seda e sua associação com o atum, não é conhecido um meio simples de reduzir as capturas acessórias sem afetar também a economia da pesca.[21]
O tubarão-seda (Carcharhinus falciformis), também conhecido por inúmeros nomes como tubarão-lombo-preto, tubarão-baleia-cinza, tubarão-oliva, tubarão-falciforme, e tubarão-foice, é uma espécie de tubarão requiem da família Carcharhinidae, nomeado pela textura suave de sua pele. É um dos tubarões mais abundantes na zona pelágica e pode ser encontrado em todo o mundo em águas tropicais. Altamente móvel e migratório, esse tubarão é encontrado com mais freqüência na borda da plataforma continental até 50 m (164 pés). O tubarão-seda tem um corpo esbelto e aerodinâmico e normalmente cresce até um comprimento de 2,5 m (8 pés 2 pol). Ele pode ser diferenciado de outros grandes tubarões requiem por sua primeira barbatana dorsal relativamente pequena com uma margem traseira curvada, sua minúscula segunda barbatana dorsal com uma ponta traseira longa e livre e suas longas barbatanas peitorais em forma de foice. É um profundo, metálico cinza-bronze acima e branco abaixo.
Com presas muitas vezes escassas em seu ambiente oceânico, o tubarão-seda é um caçador rápido, curioso e persistente. Alimenta-se principalmente de peixes ósseos e cefalópodes e é conhecido por levá-los a cardumes compactados antes de lançar ataques de boca aberta e cortante. Esta espécie geralmente arrasta cardumes de atum, uma de suas presas favoritas. Seu senso auditivo é extremamente agudo, permitindo localizar os ruídos de baixa frequência gerados por outros animais alimentadores e, por extensão, fontes de alimento. O tubarão-seda é vivíparo, o que significa que os embriões em desenvolvimento são sustentados por uma conexão placentária com a mãe. Variação geográfica significativa é vista em seus detalhes do ciclo de vida. A reprodução ocorre o ano todo, exceto no Golfo do México, onde segue um ciclo sazonal. As fêmeas dão à luz ninhadas de até 16 filhotes anualmente ou bienalmente. Os tubarões recém-nascidos passam seus primeiros meses em viveiros de recifes relativamente abrigados na plataforma continental externa, crescendo substancialmente antes de se mudarem para o oceano aberto.
O tamanho grande e os dentes cortantes do tubarão-seda o tornam potencialmente perigoso e se comporta agressivamente em relação aos mergulhadores. No entanto, os ataques são raros, pois poucos humanos entram em seu habitat oceânico. Os tubarões-seda são valorizados por suas barbatanas e, em menor grau, por sua carne, pele, óleo de fígado e mandíbulas. Devido à sua abundância, eles formam um componente importante da pesca comercial e artesanal de tubarões em muitos países. Além disso, sua associação com o atum resulta em muitos tubarões sendo capturados como capturas acessórias na pesca de atum. Embora com reprodução lenta, como a maioria dos outros tubarões, se pensava que a ampla distribuição e o grande tamanho populacional do tubarão lombo-preto protegiam as espécies contra essas pressões de pesca. No entanto, os dados agora sugerem que o número de tubarões-seda está diminuindo em todo o mundo, o que levou a IUCN a reavaliar seu status de conservação para Vulnerável em 2017.
Žralok hodvábny (lat. Carcharhinus falciformis) je druh z čeľade Carcharhinidae, pomenovaný podľa svojho jemného povrchu. Žije v tropických vodách celého sveta, najčastejšie na kraji kontinentálnych šelfov v hĺbkach do päťdesiatich metrov. Dorastá obvykle do dĺžky 2,5 metra.
V roku 2007 zmenil Medzinárodný zväz ochrany prírody jeho stupeň ohrozenia z „najmenej ohrozený“ na „takmer ohrozený“.
Žralok hodvábny (lat. Carcharhinus falciformis) je druh z čeľade Carcharhinidae, pomenovaný podľa svojho jemného povrchu. Žije v tropických vodách celého sveta, najčastejšie na kraji kontinentálnych šelfov v hĺbkach do päťdesiatich metrov. Dorastá obvykle do dĺžky 2,5 metra.
V roku 2007 zmenil Medzinárodný zväz ochrany prírody jeho stupeň ohrozenia z „najmenej ohrozený“ na „takmer ohrozený“.
Silkeshaj (Carcharhinus falciformis) är en stor haj som lever pelagiskt i tropiska och varma tempererade hav.
Arten återfinns över hela världen i tropiska och varma (> 23 °C) tempererade hav. Den lever pelagiskt men närmar sig ibland land, särskilt vid avlägsna öar. Den återfinns ned till 500 merters djup.
Denna haj har en typisk hajkropp. Den är brungrå på ovansidan och vit på undersidan, utan några speciella utmärkande drag. Det som särskiljer silkeshajen från andra hajar är bland annat att den andra ryggfenan har en lång, lös spets som nästan är 2,5 gånger fenans höjd. Den första ryggfenan sitter även längre bak än hos andra gråhajar, den börjar strax efter bröstfenorna.[1] Maxlängden är 3,3 meter, men längden är vanligtvis inte mer än 2,4 meter.
Silkeshajen är ett rovdjur som huvudsakligen äter benfiskar, men även bläckfisk och krabba.
Silkeshaj (Carcharhinus falciformis) är en stor haj som lever pelagiskt i tropiska och varma tempererade hav.
Загальна довжина досягає 2-3,5 м та максимальної ваги у 346 кг. Спостерігається статевий диморфізм: самиці більші за самців. Голова довга, округла. Тулуб довгий, обтічний. Очі середнього розміру з мигательною мембраною. На верхній щелепі є 14-16 рядків зубів та на нижній щелепі — 13-17 рядків зубів. Верхні зуби зазубрені та мають трикутну форму. Нижні зуби вузькі, прямостоячі. Зябрових щілин — 5, вони помірного розміру. У неї два спинних плавця. Перший спинний плавець знаходяться відразу після грудного. Задній спинний плавець менше за анальний плавець. Грудні плавці доволі великі, проте вузькі. Хвостовий плавець досить високий з добре розвиненою нижньою частиною. Має м'яку плакоїдну луску. Внаслідок цього акула отримала свою назву. Забарвлення коричнево-сіре, або навіть чорне.
Мешкає як в океані, так і поблизу від узбережжя. Переважно плаває у верхніх шарах, на глибині близько 10 м, може опускатися нижче — до 500 м. Дорослі акули здатні робити значні міграції. Полюбляє нагріті сонцем теплі шари води. Живиться різними видами тунцових, кефалю, кальмарами.
Це живородна риба. У виводку може бути від 2 до 16 дитинчат, зазвичай — 6-12. Акуленята народжуються досить великими (70-80 см) і абсолютно сформованими. Спочатку живуть на мілині, але вже через декілька місяців можуть відправлятися у відкрите море.
Тривалість життя сягає 20 років.
Має досить смачне м'ясо. Крім плавників і м'яса, використовується також жир з її печінки і шкіра.
Враховуючи великий розмір і форму зубів, Шовкові акули рахуються потенційно небезпечними для людини. Тим не менше вони рідко вступають в контакт, тому що надають перевагу відкритому океану. Природна цікавість і сміливість дозволяє їм підпливати до дайверів на близьку відстань, а присутність їжі може привести їх у збуджений стан і спровокувати агресію. Шовкові акули на рифах поводяться агресивніше ніж у відкритій воді. Відомі випадки коли окремі акули переслідували дайверів, яким доводилось поспіхом тікати з води. По стану на березень 2009 року в списку International Shark Attack File[1] перераховані 6 нападів, три з них неспровоковані, жоден не був летальним. Також у 2015 була зафіксована неспровокована атака[2] на Канарських островах.
Зустрічається в тропічних і субтропічних регіонах. Мешкають на заході Атлантичного океану: від штату Массачусетс в США до Бразилії. Не водяться вони лише на Карибах і в Мексиканській затоці. На сході Атлантики: від узбережжя Іспанії до північної Анголи. В Індійському океані трапляється від Мадагаскару до Шрі-Ланки, а також у Червоному морі. У Тихому океані: від Таїланду та Філіппін до Австралії й Нової Каледонії.
Carcharhinus falciformis là một loài cá trong họ Carcharhinidae. Nó là một trong các loài cá mập có nhiều ở vùng biển khơi, và có thể được tìm thấy trên khắp thế giới ở các vùng nước nhiệt đới. Tính di động cao và di cư nhiều, loài cá này thường được tìm thấy ở thềm lục địa ở độ sâu đến 50 m (164 ft). Nó có thân thon thả và thuôn và thường có độ dài 2,5 m (8 ft 2 in).
Carcharhinus falciformis là một loài cá trong họ Carcharhinidae. Nó là một trong các loài cá mập có nhiều ở vùng biển khơi, và có thể được tìm thấy trên khắp thế giới ở các vùng nước nhiệt đới. Tính di động cao và di cư nhiều, loài cá này thường được tìm thấy ở thềm lục địa ở độ sâu đến 50 m (164 ft). Nó có thân thon thả và thuôn và thường có độ dài 2,5 m (8 ft 2 in).
У шёлковой акулы тонкое и обтекаемое тело, довольно длинное, округлое рыло с едва развитой кожной складкой в передней части. Круглые глаза среднего размера оснащены мигательной перепонкой. В углах рта пролегают короткие, мелкие борозды[5][10]. Есть 14—16 и 13—17 зубных рядов с каждой стороны верхней и нижней челюстей соответственно. Верхние зубы имеют треугольную форму с сильно зазубренными краями и единственным остриём; по центру челюсти они стоят прямо, а к углам всё сильнее наклоняются. Нижние зубы узкие, прямые и с гладкими краями. У шёлковой акулы пять пар жаберных щелей средней длины[11].
Спинные и грудные плавники имеют характерную форму, по ним шёлковую акулу можно отличить от похожих видов. Первый спинной плавник относительно небольшой, размером менее 1/10 длины тела, его основание находится на одной линии со свободными концами грудных плавников. У него закруглённая, скошенная назад вершина, длина заднего свободного кончика составляет половину высоты плавника. Второй спинной плавник очень мал, меньше анального плавника, длина свободного заднего кончика почти в два раза превосходит длину плавника. Между первым и вторым спинными плавниками имеется гребень. Грудные плавники узкие и серповидные, у взрослых особей особенно длинные. У анального плавника на заднем крае над свободным кончиком имеется глубокий вырез. Хвостовой плавник довольно высок, с хорошо развитой нижней лопастью. Имеется вентральная выемка рядом с кончиком верхней лопасти. Конец верхней лопасти расположен чуть ниже кончика первого спинного плавника[5][10]. Кожа густо покрыта перекрывающимися плакоидными чешуйками. Каждая чешуйка имеет форму ромба и снабжена гребнем, оканчивающимся зубцом[7][9]. Окраска спины варьирует от золотисто-коричневого до тёмно-серого с металлическим отливом, брюхо белоснежное, белый цвет распространяется полосами на бока. Плавники (за исключением первого спинного) на концах темнее; это заметнее у молодых акул[5][9]. После смерти окраска быстро выцветает и становится серого цвета[12]. Шёлковая акула обычно достигает длины 2,5 м, максимально зарегистрированная длина и вес составляют 3,5 м и 346 кг соответственно[4]. Самки крупнее самцов[9].
Шёлковая акула распространена повсеместно в морских водах температурой выше +23 °C. В Атлантическом океане она встречается от штата Массачусетс до Испании на севере, от южной Бразилии до северной Анголы на юге, включая Средиземное море, Мексиканский залив и Карибское море. Она обитает в Индийском океане от прибрежных вод Мозамбика до Западной Австралии, в том числе в Красном море и Персидском заливе. В Тихом океане она распространена от юга Китая и Японии до южной Калифорнии и Калифорнийского залива; от Сиднея на юге до северной части Новой Зеландии и Чили[5][8]. На основании различий жизненного цикла можно вычленить четыре отдельных популяции шёлковых акул в океанических бассейнах по всему миру. Они обитают в северо-западной Атлантике, в западной и центральной частях Тихого океана, в восточной части Тихого океана и в Индийском океане[8].
Шёлковая акула в первую очередь является обитателем открытого океана: она встречается как у поверхности, так и на глубине до 200 м, но может погружаться на 500 м и более[5]. Наблюдение за акулами в восточной части Тихого океана и на севере Мексиканского залива показало, что шёлковые акулы проводили 99 % своего времени, курсируя на глубине 50 м, манера поведения оставалась неизменной независимо от времени суток[13][14]. Этот вид предпочитает находиться у континентального или островного шельфа и над расположенными в глубине коралловыми рифами. В некоторых случаях шёлковые акулы осмеливаются заходить в прибрежные воды глубиной не менее 18 м[15]. Шёлковые акулы очень подвижны и передвигаются на большие расстояния, хотя подробности их миграций малоизучены. Есть данные, что отдельные акулы проплывали до 60 км в день и покрывали расстояния до 1339 км[16]. Крупные акулы обычно перемещаются на большие расстояния, по сравнению с мелкими. В Тихом океане они проводят лето на высоких широтах, особенно в годы Южной осцилляции[17]. В Северной Атлантике большинство акул следуют на север за Гольфстримом вдоль восточного побережья США[16]. В Аденском заливе шёлковые акулы чаще встречаются в конце весны и летом[8].
Шёлковая акула входит в группу трёх наиболее распространённых пелагических акул наряду с синей и длиннокрылой акулами и считается одним из самых многочисленных видов крупных океанических животных, поголовье которого составляет как минимум десять миллионов особей[18]. В отличие от синих и длиннокрылых акул, обитающих в основном в прибрежных водах, где легче добывать пищу, шёлковые акулы предпочитают открытый океан. Шёлковая акула — это активный, любознательный и агрессивный хищник, хотя в условиях конкуренции она уступает медлительной, но более мощной длиннокрылой акуле[5]. Завидев приближение интересного объекта, шёлковая акула не выказывает пристального внимания, а медленно кружится вокруг него, иногда поворачивая голову из стороны в сторону. Тем не менее, она может реагировать с поразительной быстротой на любые изменения в её непосредственном окружении[19] . Эти акулы часто встречаются вокруг плавучих объектов, таких как лаги или привязанные морские буи[20].
Известно, что молодые шёлковые акулы образуют слабо организованные скопления, возможно, для взаимной защиты[21]. Во время миграции в стаю могут собраться более тысячи акул[22]. Как правило, группы формируются по размеру особей, а в Тихом океане, вероятно, по полу[9][23]. В группах шёлковых акул наблюдаются стычки: акулы поворачиваются боком друг к другу, разевают пасть и выпячивают жабры. Иногда они устремляются вверх, а дойдя до поверхности, меняют направление и скользят обратно на глубину. Значение такого поведения неизвестно[19]. При столкновении шёлковая акула демонстрирует угрозу — выгибает спину дугой, опускает хвост и грудные плавники и поднимает голову. Затем она начинает совершать небольшие круги, двигаясь жёстко и отрывисто и поворачиваясь боком к предполагаемой опасности[24].
Шёлковые акулы могут стать добычей крупных акул и косаток (Orcinus orca)[19]. Шёлковые акулы часто смешиваются со стаями бронзовой рыбы-молот (Sphyrna lewini), также известно, что иногда они преследуют морских млекопитающих. В Красном море зафиксирован случай, когда 25 шёлковых акул наряду с одной белопёрой и 25-ю темнопёрыми серыми акулами следовали за большой стаей афалин (Tursiops sр.). Шёлковых акул сопровождают рыбы-лоцманы (Naucrates ductor), которые «скользят» на волнах давления, производимых акулами, а также каранксы, которые подбирают остатки пищи и трутся о кожу акул, чтобы очиститься от паразитов[21][25].
Шёлковая акула — крупный хищник, питающийся в основном костистыми рыбами на всех уровнях водной толщи. В её рацион входят: тунец, макрель, сардины, кефаль, морской окунь, луциан, ставрида, скумбрия, катран, угорь, миктофовые, спинорог и рыба-ёж. Также она питается кальмарами, аргонавтами и крабами. Есть доказательства того, что шёлковые акулы объедают китовые туши[5][8]. Обилие пищи может привлечь шёлковых акул в большом количестве. В подобных скоплениях в Тихом океане зафиксировано, как акулы сбивали косяк мелких рыбок в компактный шар и блокировали его у поверхности воды, после чего поглощали добычу[8]. Атакуя плотный шар, шёлковые акулы с разинутой пастью проплывали сквозь него, а рыбки застревали у них в углах челюстей. Несмотря на то, что одновременно может кормиться несколько особей, каждая из них начинает свою атаку самостоятельно[21].
Исследования, проведённые у побережья Флориды и Багамских островов, показали, что шёлковые акулы очень чувствительны к звукам, в частности, низкочастотным (10—20 Гц) с нерегулярными импульсами. В ходе экспериментов эти звуки привлекали акул за сотни метров. Шёлковые акулы ориентируются на эти звуки, потому что они похожи на шум, создаваемый при кормлении животных, таких как птицы или дельфины, и таким образом указывают на перспективные источники питания[19][21]. Эти исследования также показали, что привлечённые определённым звуком шёлковые акулы обращаются в бегство, если этот звук резко изменяет амплитуду. После неоднократного воздействия шёлковые акулы привыкают к изменениям, хотя этот процесс занимает гораздо больше времени, по сравнению с более смелыми белопёрыми серыми акулами[19]. У них хорошо развито обоняние — они реагируют на экстракт рыбы, разведённой в воде в пропорции 1:10 млрд[26]
Сила давления при укусе шёлковой акулы длиной 2 м составляет 890 ньютонов[27]. Существует устойчивая связь между этим видом акул и тунцами. У берегов Ганы почти каждая стая тунцов сопровождается шёлковыми акулами, а в восточной части Тихого океана эти акулы наносят такой ущерб орудиям лова, что рыбаки дали им прозвище «пожиратели сетей»[5][12]. Охотясь на один и тот же косяк рыб, шёлковые акулы и бутылконосые дельфины конкурируют друг с другом. Количество рыбы, съедаемой дельфинами, уменьшается при увеличении числа присутствующих акул. Когда акул много, они имеют тенденцию оставаться внутри косяка, в то время как дельфины держатся на периферии, возможно, чтобы избежать случайных травм во время нападения акул на рыб. И наоборот, если собирается достаточно большая группа дельфинов, они могут отогнать акул от добычи. Независимо от того, кто доминирует, два хищника не выказывают никакой открытой агрессии по отношению друг к другу[28].
Подобно другим представителям рода серых акул, шёлковые акулы являются живородящими. Опустев, желточный мешок превращается в своеобразную плаценту, через которую мать обеспечивает зародыш питанием. По сравнению с другими живородящими акулами плацента шёлковых акул меньше похожа на плаценту млекопитающих, поскольку между тканями плода и матери нет смыкания. Кроме того, эритроциты плода намного меньше материнских. Взрослые самки имеют один функциональный яичник (справа) и две функциональные матки, которые разделены вдоль на отдельные отсеки для каждого эмбриона[29].
Полагают, что шёлковая акула в большинстве частей света размножается круглый год, в то время как в Мексиканском заливе спаривание и роды проходят в конце весны или начале лета (май — август)[15]. Беременность длится 12 месяцев. Самки приносят потомство ежегодно либо каждые два года[8]. В помёте от 1 до 16 акулят, как правило — 6—12. Детёныши рождаются на рифовой кромке континентального шельфа, где достаточно корма и нет крупных пелагических акул. За первый год жизни акулята вырастают на 25—30 см. Через несколько месяцев молодые акулы мигрируют из места рождения в открытый океан[21][23].
Жизненный цикл шёлковых акул зависит от места обитания. Акулы Северо-Западной Атлантики, как правило, во всех возрастных группах крупнее своих сородичей, обитающих в западно-центральной части Тихого океана, в то время как акулы восточной части Тихого океана обычно меньше акул в других регионах[8].
В целом у шёлковых акул умеренный темп роста по сравнению с другими видами акул, хотя он значительно варьируется в индивидуальном порядке. Одно исследование, проведённое в центральной части Тихого океана, показало, что самки растут гораздо медленнее самцов[15]. Максимальные темпы роста отмечаются у акул на севере Мексиканского залива, а минимальные — у акул, обитающих у северо-восточных берегов Тайваня[30] . Самцы и самки достигают половой зрелости в возрасте 6—10 лет и 7—12 лет соответственно[8]. Акулы, населяющие умеренные воды, могут расти медленнее и позже созревать по сравнению с обитателями жаркого климата[31]. Максимальный срок жизни составляет не менее 22 лет[18].
Учитывая крупный размер и форму зубов, шёлковые акулы считаются потенциально опасными для человека. Тем не менее, они редко вступают в контакт с людьми, поскольку предпочитают жить в открытом океане[9]. Природное любопытство и смелость позволяют им подплывать к дайверам на близкое расстояние, а присутствие пищи может привести их в возбуждение и спровоцировать агрессивное поведение. Шёлковые акулы на рифах ведут себя агрессивнее, чем в открытой воде. Известны случаи, когда отдельные акулы преследовали дайверов, которым приходилось спешно вылезать из воды[25]. По состоянию на май 2009 года в списке International Shark Attack File перечислены шесть нападений, приписываемых шёлковым акулам, три из них были неспровоцированными и ни одно не имело летального исхода[32].
Шёлковые акулы являются объектом промышленного и любительского рыболовства. Их добывают у берегов Мексики, Гватемалы, Сальвадора, Коста-Рики, США, Эквадора, Испании, Португалии, Шри-Ланки, Мальдивских островов, Йемена и Кот-д'Ивуара. Большее число акул случайно попадается в сети во время ярусного промысла тунца и в кошельковые неводы по всему ареалу. Это наиболее добываемый в качестве прилова вид акул в восточной части Тихого океана и Мексиканского залива и второй по численности (после синей акулы) в целом[8][33]. Их плавники ценятся как ингредиент для супа из акульих плавников: часто у пойманных акул отрезают плавники, а тушу выбрасывают в море. Ежегодно в мире продаются плавники от половины до полутора миллионов шёлковых акул, это второй или третий наиболее продающийся на рыночном аукционе в Гонконге вид плавников, что составляет более половины мирового рынка[8]. Мясо продают свежим или высушенным и засоленным, также используют кожу, жир[5] и челюсти: этот вид является основным источником сувенирных челюстей, которые покупают в тропиках туристы[21]. Шёлковая акула является объектом спортивного рыболовства[9].
В Северной Каролине были найдены ископаемые остатки зубов шёлковой акулы, предположительно относящиеся к периоду от миоцена до плиоцена-голоцена[34][35]. Ископаемые зубы были также обнаружены в слоях эпохи плиоцена в карьере в Тоскане, Италия[36]. Ископаемые зубы Carcharhinus elongatus, раннего представителя своей линии с гладкой поверхностью зубов, находили в слоях эпохи олигоцена в Вирджинии и Южной Каролине. Набор зубов эпохи эоцена из Египта также напоминает зубы этого вида акул[35].
Первоначальные попытки определить эволюционное положение шёлковой акулы не принесли результата. На основании морфологии Джек Гаррик в 1982 году предположил, что ближайшим родственным видом является серая акула Сейла (Carcharhinus sealei)[37]. В 1988 году Леонард Компаньо поместил шёлковую акулу в неофициальную «переходную группу», к которой также относятся черноносая акула (Carcharhinus acronotus), мальгашская ночная акула (Carcharhinus melanopterus), пугливая акула (Carcharhinus cautus), узкозубая акула (Carcharhinus brachyurus) и кубинская ночная акула (Carcharhinus signatus)[38].
В 1992 году Гэвин Нейлор на основании филогенетического анализа обнаружил, что шёлковая акула является частью группы, в которую входят крупные виды акул, у которых есть гребень между первым и вторым спинными плавниками. К одной из ветвей этой группы принадлежат серо-голубая акула (Carcharhinus lumbeus) и большеносая акула (Carcharhinus altimus), тогда как шёлковая акула является базальным членом другой ветви и таксоном, родственным кладе, в которую входят карибская рифовая акула (Carcharhinus perezi), галапагосская акула (Carcharhinus galapagensis), длиннокрылая акула (Carcharhinus longimanus), тёмная акула (Carcharhinus obscurus) и синяя акула (Prionace glauca)[39]. В 2008 году анализ ДНК подтвердил близкое родство шёлковой, синей и большеносой акул[40].
Считалось, что, будучи самым многочисленным и широко распространённым видом акул на Земле, шёлковая акула не подвергается опасности уничтожения, несмотря на то, что огромное количество акул гибнет в рыболовецких сетях. В 1989 году в южной и центральной частях Тихого океана в ходе ярусного промысла тунца было добыто около 900 000 особей, но это не оказало существенного влияния на общую численность популяции[18]. Данные по добыче этой акулы зачастую неточны из-за затруднённой идентификации. Тем не менее, появляется всё больше доказательств того, что численность шёлковых акул существенно снизилась по всему миру, так как невысокий репродуктивный уровень не в состоянии поддерживать её стабильность при такой интенсивной добыче. По сообщению Продовольственной и сельскохозяйственной организации ООН (ФАО), общий годовой вылов снизился с 11 680 тонн в 2000 году до 4358 тонн в 2004 году. Региональные данные обнаруживают схожую тенденцию, оценивая снижение приблизительно на 90 % в центральной части Тихого океана за период с 1950 по 1990 год, на 60 % у берегов Коста-Рики с 1991 по 2000 год, на 91 % в Мексиканском заливе с 1950 по 1990 год и на 85 % (всех крупных серых акул) в северо-западной Атлантике с 1986 по 2005 год. У берегов Шри-Ланки добыча шёлковой акулы сократилась с пикового значения 25 400 тонн в 1994 году до 1960 тонн в 2006 году, что свидетельствует о коллапсе местного рынка. С другой стороны, по данным японских рыбаков, в Тихом и Индийском океанах не отмечалось никаких изменений добычи между 70-ми и 90-ми годами минувшего столетия, а обоснованность методов, используемых для оценки снижения в Мексиканском заливе и в северо-западной Атлантике, вызывает много споров[41][42][43].
В свете последних результатов в 2007 году Международный союз охраны природы (МСОП) изменил статус сохранения шёлковой акулы на «Близкий к уязвимому положению» по всему миру. На региональном уровне этому виду присвоен статус «Уязвимый» (VU) в восточной и юго-восточной частях Тихого океана и в северо-западной и западной частях Центральной Атлантики. Позитивное влияние на численность популяции этого вида должен оказать запрет на срезание плавников, принятый в США, Австралии и Европейском Союзе. Такие организации, как Международная комиссия по сохранению атлантических тунцов и Межамериканская комиссия по сохранению тропического тунца, также предприняли шаги для улучшения мониторинга рыболовства с конечной целью снижения прилова акул[8]. Однако, учитывая тот факт, что шёлковые акулы являются мигрирующим видом, связанным с тунцами, простого способа снижения прилова не существует[44].
У шёлковой акулы тонкое и обтекаемое тело, довольно длинное, округлое рыло с едва развитой кожной складкой в передней части. Круглые глаза среднего размера оснащены мигательной перепонкой. В углах рта пролегают короткие, мелкие борозды. Есть 14—16 и 13—17 зубных рядов с каждой стороны верхней и нижней челюстей соответственно. Верхние зубы имеют треугольную форму с сильно зазубренными краями и единственным остриём; по центру челюсти они стоят прямо, а к углам всё сильнее наклоняются. Нижние зубы узкие, прямые и с гладкими краями. У шёлковой акулы пять пар жаберных щелей средней длины.
Спинные и грудные плавники имеют характерную форму, по ним шёлковую акулу можно отличить от похожих видов. Первый спинной плавник относительно небольшой, размером менее 1/10 длины тела, его основание находится на одной линии со свободными концами грудных плавников. У него закруглённая, скошенная назад вершина, длина заднего свободного кончика составляет половину высоты плавника. Второй спинной плавник очень мал, меньше анального плавника, длина свободного заднего кончика почти в два раза превосходит длину плавника. Между первым и вторым спинными плавниками имеется гребень. Грудные плавники узкие и серповидные, у взрослых особей особенно длинные. У анального плавника на заднем крае над свободным кончиком имеется глубокий вырез. Хвостовой плавник довольно высок, с хорошо развитой нижней лопастью. Имеется вентральная выемка рядом с кончиком верхней лопасти. Конец верхней лопасти расположен чуть ниже кончика первого спинного плавника. Кожа густо покрыта перекрывающимися плакоидными чешуйками. Каждая чешуйка имеет форму ромба и снабжена гребнем, оканчивающимся зубцом. Окраска спины варьирует от золотисто-коричневого до тёмно-серого с металлическим отливом, брюхо белоснежное, белый цвет распространяется полосами на бока. Плавники (за исключением первого спинного) на концах темнее; это заметнее у молодых акул. После смерти окраска быстро выцветает и становится серого цвета. Шёлковая акула обычно достигает длины 2,5 м, максимально зарегистрированная длина и вес составляют 3,5 м и 346 кг соответственно. Самки крупнее самцов.
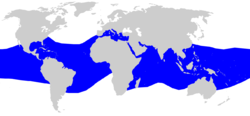
絲鯊(學名:Carcharhinus falciformis)又名鐮狀真鯊、平滑白眼鮫、黑鯊,為真鯊屬的一種。
絲鯊分佈於全球的熱帶和亞熱帶溫水海洋中(所謂溫水即高於攝氏23度的水),一般出現於大西洋、太平洋和印度洋。在西大西洋,牠們分佈於從麻薩諸塞州到巴西的地區,而在東大西洋,牠們分佈於從西班牙到安哥拉的地區。在印度洋,牠們出現於紅海、馬達加斯加、馬爾地夫、斯里蘭卡和西澳大利亞等的地區。在太平洋,牠們出現於由中國到新西蘭的地區及從下加利福尼亞州到祕魯的地區。[2]
絲鯊一般生活在遠洋,但有時亦會在海岸附近出現,尤其是人跡罕至的海島的海岸線。牠們最少也會在水底500米。
最初在1839年,约翰内斯·彼得·米勒(Johannes Peter Müller)和弗里德里希·古斯塔夫·雅各布·亨勒(Friedrich Gustav Jakob Henle)把絲鯊命名為Carcharias (Prionodon) falciformis,而在其他文獻中出現的其他名稱有Squalus或Prionodon tiburo、Gymnorhinus或Gymnorrhinus pharaonis、Aprionodon sitankaiensis、Carcharhinus floridanus、Eulamia malpeloensis及Carcharhinus atrodorsus。[2]
絲鯊為遠洋區數量最豐富的鯊魚之一。絲鯊具有遷徙、洄游的習性,常見於大陸棚邊緣深至50公尺(164英尺)處;魚身流線型,身長普遍可達2.5公尺(8英尺2英寸),上半為帶有金屬色澤的深青銅灰、下半白色。絲鯊的第一背鰭相對其他鯊魚較為小型、連接背緣曲線,第二背鰭嬌小、帶有背部尖突,胸鰭大型、鐮刀狀。絲鯊的體型龐大,皮膚像絲一般嫩滑。牠們的背部呈棕色或灰色,腹部呈白色,沒有什麼特別的斑駁。牠們的第二個背鰭尖端非常長,約是鰭本身長度的2.5倍。
牠們最大可長達3.3米,但是一般的絲鯊都不會長超於2.4米。
絲鯊比另外兩種大型的遠洋鯊魚大青鯊和遠洋白鰭鯊要來得活躍和具更少侵略性。牠們比大青鯊和遠洋白鰭鯊更接近海岸,但也是屬於遠洋鯊魚,因為接近海岸的絲鯊也不是太常見。
絲鯊並不是常常都對潛水人構成威脅,但若潛水人用魚叉攻擊其他魚而令這些魚流出血來的話,牠們就會聞到這些血味,變得極具攻擊性,因此人們把牠們列為有潛在危險的鯊魚。
絲鯊主要是進食海岸附近或遠洋的多骨魚類,但是牠們也會進食烏賊及遠洋的蟹。
絲鯊是胎生動物,從2到14歲期間,牠們的幼鯊會不斷成長。在出生時,牠們長約80公分。[3] 在性成熟後,雌性絲鯊長約2.2米,雄性則長約2米。
為高經濟價值的食用魚,肉質佳,適合製成各式肉品。魚肝可製成魚油,皮可製成皮革。
 保全状況評価[1] VULNERABLE
保全状況評価[1] VULNERABLE 分類 界 : 動物界 Animalia 門 : 脊索動物門 Chordata 綱 : 軟骨魚綱 Chondrichthyes 目 : メジロザメ目 Carcharhiniformes 科 : メジロザメ科 Carcharhinidae 属 : メジロザメ属 Carcharhinus 種 : クロトガリザメ C. falciformis 学名 Carcharhinus falciformis (Müller & Henle, 1839) シノニム
分類 界 : 動物界 Animalia 門 : 脊索動物門 Chordata 綱 : 軟骨魚綱 Chondrichthyes 目 : メジロザメ目 Carcharhiniformes 科 : メジロザメ科 Carcharhinidae 属 : メジロザメ属 Carcharhinus 種 : クロトガリザメ C. falciformis 学名 Carcharhinus falciformis (Müller & Henle, 1839) シノニム 
クロトガリザメ (Carcharhinus falciformis) は、メジロザメ属に属するサメの一種。全世界の熱帯の外洋域で見られ、最も個体数の多い外洋性サメの一つである。深度50mまでの表層を高速で回遊する。体は細い流線型で、通常2.5m程度。第一背鰭が小さく、第二背鰭の後端が長く伸び、鎌型の長い胸鰭を持つことが特徴である。背面は金属光沢のある灰褐色で、腹面は白い。皮膚は非常に滑らかに見え、ここから英名 "Silky shark" が付けられた。
高速で粘り強い捕食者で、主に硬骨魚や頭足類を食べる。連携して小魚の群れを襲うことや、好物であるマグロを追って長距離を移動することがある。胎生で年中繁殖し、雌は1-2年ごとに16匹までの仔を産む。幼体は大陸棚の岩礁で成長した後、外洋へ出て行く。大きさと歯の形状から、ヒトに対して潜在的に危険だと考えられるが、外洋性のために遭遇する機会は少ない。個体数が多いことからフカヒレなどを目的に大量に漁獲され、マグロ漁による混獲数も多い。個体数は急激に減少しており、IUCNは保全状況を準絶滅危惧としている。
ドイツの生物学者ヨハネス・ペーター・ミュラーとヤーコプ・ヘンレによる1839年の著作 Systematische Beschreibung der Plagiostomen において、Carcharias (Prionodon) falciformis の名で記載された。その後本種はCarcharhinus 属に移された[3][4]。ミュラーとヘンレがキューバから得られた53cmの胎児をタイプ標本に用いたため、成体は長年に渡って別の種として扱われ、1943年にはHenry Bigelow・William Schroeder・Stewart SpringerによってCarcharhinus floridanus として記載文が発表された。1964年に、Jack Garrick・Richard Backus・Robert Gibbs, Jr.によってC. floridanus はC. falciformis のシノニムとされた[5]。
種小名 falciformis はラテン語で"鎌型"を意味し、背鰭と胸鰭の形に因んだものである[2]。英名silky sharkは、小さく密な皮歯によって、他のサメより皮膚の質感が繊細であることに由来する[6]。他の英名として、blackspot shark(通常はCarcharhinus sealei を指す)・grey reef shark(通常はオグロメジロザメを指す)・grey whaler shark・olive shark・reef shark・ridgeback shark・sickle shark・sickle silk shark・sickle-shaped shark・silk shark・silky whalerがある[7]。
ノースカロライナ州の2個体のヒゲクジラの近く(一つは更新世-完新世(約12,000年前)の泥岩、もう一つはグースクリークの後期鮮新世(約3500万年前)の石灰岩)と、プンゴ川の中新世(2300-530万年前)の層[8][9]、イタリア・トスカーナ州のCava Serredi採石場の鮮新世の地層から歯の化石が発見されている[10]。さらに、滑らかな歯を持った、本種の系統の古い代表種である Carcharhinus elongatus が漸新世(3400-2300万年前)のバージニア州のOld Church層・サウスカロライナ州のAshley層から得られており、記載が不十分ではあるが、エジプトの始新世(5600-3400万年前)の層からも、この種に似た一揃いの歯が知られている[9]。
初期の形態系統解析ではCarcharhinus sealei と近縁だとされ[11]、1988年には、ハナグロザメ・ツマグロ・Carcharhinus cautus ・クロヘリメジロザメ・ナガハナメジロザメとともに"transitional group"と命名された群に含められた[12]。1992年の分子系統解析では、ペレスメジロザメ・ガラパゴスザメ・ヨゴレ・ドタブカ・ヨシキリザメを含むクレードの姉妹群に位置づけられた[13][14]。2012年の包括的な解析結果ではこれと異なる系統樹が描かれているが、ヨシキリザメと近縁であることについては一貫した結果が得られている[15]。
汎存種であり、水温23℃以上の海域で見られる。大西洋では、北限はマサチューセッツ州-スペイン、南限はブラジル南部-アンゴラ北部。地中海・メキシコ湾・カリブ海にも分布する。インド洋では、南限はモザンビーク-西オーストラリアで、紅海・ペルシャ湾でも見られる。太平洋では、北限は中国南部・日本-バハカリフォルニア南部・カリフォルニア湾、南限はシドニー・ニュージーランド北部-チリ北部[2][3]。生活史の差異から、北西大西洋、西部・中央太平洋、東部太平洋、インド洋の4つの個体群が識別される[2]。
主に外洋性で、最深で500m以上潜る可能性はあるが、海面から深度200mまででよく見られる[3]。東部太平洋とメキシコ湾北部でのデータロガーによる調査では、99%の時間を海面から深度50mまで、80–85%の時間を水温26-30℃の場所で過ごしていた。このパターンは昼夜を問わず見られた[16][17]。大陸棚の縁を好み、深い岩礁の上や島の周辺でよく見られる。分布域は外洋よりも、大陸の縁に沿って長く南北に伸びる。稀に、水深18m程度の浅い沿岸に出没することもある[18]。移動性が高く回遊を行うが、その詳細はあまり分かっていない。データロガーによる調査では、1日に60km、調査期間中の総計で最大1339kmを移動した[19]。大型個体は小型個体より長距離を移動する。少なくとも太平洋では、夏には少し高緯度に移動し、特にエルニーニョによる暖水塊が存在する場合は顕著である[20][21]。北大西洋では、ほとんどの個体がアメリカ合衆国東海岸に沿って、メキシコ湾流に乗って北に移動する[19]。アデン湾では、移動は晩春から夏に起こる[2]。
体は細く流線型、吻は丸く、かなり長い。鼻孔の前鼻弁はあまり発達しない。眼は中程度の大きさで丸く、瞬膜を備える。口角の唇褶は短く浅い[3][22]。片側の歯列は、上顎で14–16、下顎で13–17。典型的には両顎共に15である。上顎歯は三角形で強い鋸歯を持ち、後縁には凹みがある。中央の歯は直立するが、口角に近づくほど傾く。下顎歯は細く直立し、縁は滑らかである。鰓裂は5対で中程度の長さ[23]。
背鰭と胸鰭は独特で、本種を同定する助けとなる。第一背鰭は比較的小さく、高さは個体の全長の1/10に満たず、胸鰭の後端より後ろから起始する。先端は丸く、後縁はS字、遊離した後端の長さは高さの半分程度。第二背鰭は微小で、臀鰭より小さく、鰭の高さの3倍近い長い遊離端を持つ。背鰭の間には細い隆起線が走る。胸鰭は細く鎌型で、成体では特に長い。臀鰭は第二背鰭の少し前より起始し、後縁は深く凹む。尾鰭はかなり高く、下葉はよく発達する[3][22]。
皮膚は細かく重なり合った皮歯に密に覆われる。各皮歯は菱形で、前縁から後縁まで水平隆起線を持つ。線の数は個体の成長とともに増える[5][6]。背面は金属光沢のある金褐色から暗灰色。腹面は白で、体側にいは微かな淡い帯が伸びる。第一背鰭以外の鰭は先端が黒くなり、若い個体では特に顕著である[3][6]。体色は死後に急速に薄れ、鈍い灰色となる[24]。メジロザメ属内では大型で、通常は2.5m程度だが、最長で3.5m、最重で346kgの記録がある[7]。雌は雄より大きくなる[6]。
個体数は数千万と見積もられ、世界の大型海洋生物の中で最も個体数が多いものの一つだと推定される。ヨシキリザメ・ヨゴレとともに、外洋で最もよく見られるサメの一つでもある[25]。他の2種と比べ、本種は厳密に外洋性というわけではなく、真の外洋よりも餌が容易に得られる陸地に近い沖合に最も多い傾向がある。活動的で好奇心が強く、攻撃的な捕食者である。だが、餌を巡る争いでは、本種より泳ぎが遅いがより攻撃的であるヨゴレには敵わない[3]。興味あるものに近づいた場合、本種は頭を振りながらゆっくりとその周りを旋回するが、周囲の状況が変化した場合は非常に速く応答する[26]。流木やブイなどの浮遊物の周辺でよく見られる[27]。
若い個体はおそらく身を守るため、緩く大きな群れを作ることが知られる[28]。回遊中には1千匹を超える個体が集合することがある[29]。これらの群れは個体サイズごとに分かれており、太平洋ではおそらく性別によっても分離している[6][20][30]。群れの内部では、互いに体を斜めにして体側を見せつけあう、口や鰓を広げる、などの行動が観察されている。時折、突如垂直に浮上し、水面に達する直前に方向転換して戻っていくという行動も見られる。これらの行動の意義は明らかではない[26]。脅威に曝されると威嚇行動を行う。背を弓なりに曲げ、尾と胸鰭を下ろして頭を持ち上げた姿勢をとり、強張った動きで体側を相手に向けながら小さなループを描いて泳ぐ[31]。
大型のサメやシャチが潜在的な捕食者として挙げられる[32]。寄生虫として、ウミクワガタ類の Gnathia trimaculata[33]、カイアシ類の Kroeyerina cortezensis[34]、条虫のDasyrhynchus variouncinatus ・Phyllobothrium 属の一種[35][36]などがある。頻繁にアカシュモクザメとの混群を作り、また、海獣に付いていくことも知られている。紅海でのある記録では、25匹の本種に加え、25匹のオグロメジロザメ、1匹のツマジロがハンドウイルカ属の大きなポッドに追従していた。本種自体に追従する魚類として、サメの前方の圧力波に乗るブリモドキの幼体や、餌の断片を拾ったり体を擦り付けて寄生虫を落としたりするアジ科魚類などがある[28][37]。
日和見的な捕食者である。餌は主に遊泳性から底生の硬骨魚で、マグロ・サバ・イワシ・ボラ・ハタ・フエダイ科・ムロアジ属・イスズミ科・ハマギギ科・ウナギ・ハダカイワシ・カワハギ科・モンガラカワハギ科・ハリセンボンが含まれる。イカ・カイダコ・ワタリガニ科なども食べる可能性があり、化石記録からはクジラの死骸を漁ることも推測される[2][3][8]。複数個体が連携して効率よく狩りをすることも知られる。その一例として、太平洋で、小魚の群れを取り囲んで密な塊とし(ベイト・ボール)、これを水面に追い詰めて群れ全体を捕食することが記録されている[2]。捕食時には、その塊に突入し、顎を開いたまま通り抜けて顎の端で獲物を引っ掛ける。複数個体が一度に突入することはあるが、各攻撃は独立して行われる[28]。
フロリダとバハマでの研究から、本種は音、特に10–20 Hzの低周波音や不規則なパルスに敏感であることが示された。実験では、これらの音は数百mの距離から本種を惹き寄せた。この音は鳥やイルカが獲物を捕食するときの音に似ており、自然下では獲物の存在を意味するものと考えられる[26][28]。これらの研究では、音の振幅や特性が変化した場合、それが別の捕食者によるものであるかどうかに関わりなく、本種は反転して離れていくことも示された。繰り返し音の変化に暴露することで馴化を起こして近づいてくるようになるが、より大胆なヨゴレと比べ、これにかかる時間は長い[32]。
咬合力は、2mの個体で890Nと測定されている[38]。本種はマグロに強く引き寄せられる。ガーナではほぼ全てのマグロの群れが後方に本種を伴っており、東太平洋では捕えたマグロやその漁具を破壊するため、漁業者から"net-eating shark"と呼ばれる[3][24]。本種とハンドウイルカが同じ群れを獲物として競合することがあり、イルカが食べられる分は本種が食べる分だけ減少する。サメの数が多い場合、イルカは群れの辺縁に留まり、サメの捕食行動による偶発的な傷害を避ける傾向にある。逆にイルカが十分に多い場合、獲物の群れから本種は追い払われる。どちらが勝利したかにかかわらず、この2種の捕食者はお互いに対して明白な攻撃行動に及ぶことはない[39]。
他のメジロザメ類同様に胎生で、卵黄を使い果たした胎児は卵黄嚢を胎盤に転換する。他の胎生のサメと比較し、本種の胎盤は哺乳類のものとの共通点が少なく、胎児と母体の組織が相互に嵌合していない。さらに、哺乳類とは逆に、胎児の方が母体より赤血球が小さい。成体雌は右側の卵巣と左右の子宮が機能し、子宮内は胚が1個ずつ収められる区画に分かれている[40]。
ほとんどの場所では年中繁殖すると見られるが、メキシコ湾では交尾と出産は晩春か初夏(5-8月)に起こる[18][30]。だが、データのバイアスによって、繁殖に見かけ上の季節性が見られているだけである可能性もある[2]。妊娠期間は12ヶ月で、毎年、または2年毎に繁殖する[1]。産仔数は1-16(典型的には6-12)で、母体の大きさにつれて増加する[2]。大型の外洋性サメを避けられることと、十分な餌が得られることから、大陸棚外縁の岩礁を幼体の成育場として用いる。捕食の危険性を減らすため幼体の成長は早く、初年度には25-30cm成長する。数ヶ月後(メキシコ湾では最初の冬)には成育場を離れて外洋に移動する[2][28][30]。
出生時・性成熟時の大きさ 地域 出生時 性成熟(雄) 性成熟(雌) 北西大西洋 68-84cm[2] 2.15-2.25m[25] 2.32-2.46m[25] 東部大西洋 ? 2.20m[41] 2.38-2.50m[24][41] インド洋 56-87cm[2] 2.39-2.40m[2][42] 2.16-2.60m[2][42] 西部太平洋 ? 2.10-2.14m[43][44] 2.02-2.20m[43][45] 中部太平洋 65-81cm[45] 1.86m[46] 2.00-2.18m[20][46] 東部太平洋 70cm[2] 1.80-1.82m[1][2] 1.80-1.82m[1][2]生活史の詳細は地域により異なる(表を参照)。北西大西洋では中部・西部太平洋より大きく、東部太平洋では他の地域より小さい傾向がある。東部大西洋とインド洋では北西大西洋より大きいようだが、調査個体数が少ないため更なるデータが必要である[2]。
全体的な成長速度で見れば、本種は他のサメと同程度で、雌雄の成長速度も似ている。だが、個体差はかなり大きい。中部太平洋での調査では、雌は雄より成長が遅いとされたが、このデータには大型の雌が含まれていないため結果が不正確となっているかもしれない[18]。報告された中で、最も成長が速いのはメキシコ湾北部、最も遅いのは台湾北東部である[45]。雄は6-10歳・雌は7-12+歳で性成熟する[2]。温帯域の個体は熱帯域の個体より成長・性成熟が遅い[45]。寿命は最低でも22年[25]。
その大きさと歯の形から、潜在的に危険だと考えられているが、外洋性であるため人と接触することは少ない[6]。好奇心が強く大胆であるためダイバーに繰り返し近づき、餌の存在下では危険なほど興奮する。外洋よりも岩礁域での遭遇ではより攻撃的になる傾向がある。各個体が持続的にダイバーに圧力をかけ、最終的に水中から追い出したケースもある[37][47]。 2009年の国際サメ被害目録は6件の攻撃を記録しており、その内3件が非挑発例で死亡例はない[48]。
メキシコ・グアテマラ・エルサルバドル・コスタリカ・米国・エクアドル・スペイン・ポルトガル・スリランカ・モルディブ・イエメン・コートジボワールで行われる、複数種のサメを対象とした商業漁業・地域漁業で大量に漁獲されている。さらに、これより多数が分布域全域でのマグロ延縄・巻網で混獲され、魚群収集装置 (FAD) が用いられた場合は特に多い。東太平洋とメキシコ湾では最も混獲される量の多いサメで、全世界でもヨシキリザメに次いで2番目である[2][49]。鰭はフカヒレの材料とされ、鰭のみを切り取って体は海上で捨てるフィニングも行われる。鰭は年間50-150万個体が取引されていると見られる。香港のフカヒレ市場では2-3番目に多い種で、全世界では取引量の半分以上を占めている[1][2]。肉は生・干物・塩漬けで販売され、鮫皮・肝油も利用される[3]。また、熱帯で骨董品として観光客に販売されるサメの顎標本の主な供給源ともなっている[28]。遊漁者にも捕獲される[6]。
地球上で最も豊富で分布域の広いサメとして、漁獲による死亡率の高さにもかかわらず、一時は乱獲に耐性があると考えられていた。1989年だけで、90万個体が太平洋南部・中部でのマグロ延縄漁によって混獲されたが、一見して個体数には影響はないように見える[25]。本種の漁獲データは、種レベルでの混同や疑問のある同定による過小報告によって混乱したものとなっている。それでも、本種の繁殖力はそれほど高くないためこのレベルの乱獲には耐えられず、実際には全世界で減少していることを示す多数の証拠がある。FAOによる全世界での年間漁獲量は、2000年の11,680 tから2004年には4,358 tに減少している。地域的にも傾向は同じで、中部太平洋では1950年代-1990年代で90%・コスタリカでは1991-2000年で60%・メキシコ湾では1950年代-1990年代で91%・北西大西洋では(他の大型メジロザメ類を含め)1950年代-1990年代で85%減少したと見られている。スリランカでは、1994年の25,400 tをピークとして2006年には1,960 tに減少したと報告されており、資源が枯渇したと見られる。対照的に、太平洋とインド洋での日本の漁業では1970年代-1990年代の間で変化は見られず[1]、メキシコ湾と北西大西洋の資源量評価に用いた方法論の妥当性についても多くの議論がある[50][51][52]。
最新の知見から、2007年にIUCNは本種の全世界での保全状況を軽度懸念から準絶滅危惧に変更した。大西洋南西部・インド洋・太平洋中西部では同じく準絶滅危惧、太平洋中東部・南東部、大西洋北西部・中西部では危急種とされている。海洋法に関する国際連合条約では高度回遊性魚種として附属書Iに載せられているが、特別な保護政策は取られていない。多くの国家や超国家的機関により進められている、フィニング禁止活動は本種の保護に繋がる[1]。大西洋まぐろ類保存国際委員会 (ICCAT) や全米熱帯まぐろ類委員会 (IATTC) のような機関も、サメの混獲を減らすことを最終目標とした漁業監視の改善措置を講じている[2]。だが、マグロを追って広範囲を回遊する習性から、水産活動に影響を与えずに混獲を減らすのは容易ではないと考えられる[21]。
|date= (help)CS1 maint: Explicit use of et al.
クロトガリザメ (Carcharhinus falciformis) は、メジロザメ属に属するサメの一種。全世界の熱帯の外洋域で見られ、最も個体数の多い外洋性サメの一つである。深度50mまでの表層を高速で回遊する。体は細い流線型で、通常2.5m程度。第一背鰭が小さく、第二背鰭の後端が長く伸び、鎌型の長い胸鰭を持つことが特徴である。背面は金属光沢のある灰褐色で、腹面は白い。皮膚は非常に滑らかに見え、ここから英名 "Silky shark" が付けられた。
高速で粘り強い捕食者で、主に硬骨魚や頭足類を食べる。連携して小魚の群れを襲うことや、好物であるマグロを追って長距離を移動することがある。胎生で年中繁殖し、雌は1-2年ごとに16匹までの仔を産む。幼体は大陸棚の岩礁で成長した後、外洋へ出て行く。大きさと歯の形状から、ヒトに対して潜在的に危険だと考えられるが、外洋性のために遭遇する機会は少ない。個体数が多いことからフカヒレなどを目的に大量に漁獲され、マグロ漁による混獲数も多い。個体数は急激に減少しており、IUCNは保全状況を準絶滅危惧としている。
미흑점상어(학명: Carcharhinus falciformis)는 흉상어목 흉상어과에 속하는 물고기이다. 몸길이는 3.3m로서 상어에서는 중형인 어류에 속한다.
미흑점상어는 날씬한 방추형의 몸매와 유선형의 몸매를 가진 어종으로 영어권에서는 실키 샤크(Silky Shark)라는 이름으로도 불리는 물고기이다. 이동성이 뛰어나며 철새와 같이 계절에 따라서 서식지를 이동하는 회유성 어종에 속하고 등지느러미가 2개인 물고기이다. 2개의 등지느러미는 곡선 후방 작은 미진이 있는 제1등지느러미와 꼬리지느러미와 비교적 가까이 있고 작은 제2등지느러미를 가지고 있다. 두번째의 등색 핀과 길고 낫모양의 가슴핀을 가지고 있으며 이것을 통해 다른 레퀴엠의 상어와 구분을 하기도 한다. 옆줄을 토대로 등쪽은 짙은 청동색을 하고 있으며 배쪽은 흰색을 띄고 있다. 가끔 사람을 공격한 사례도 있지만 황소상어와는 달리 사람에게 크게 위협적인 상어는 아니며 호기심이 많은 상어이기도 하다. 또한 위턱과 아래턱에 날카로운 이빨들이 줄지어 나 있다. 먹이로는 멸치, 청어, 꽁치, 고등어, 숭어 등의 어류들과 갑각류, 오징어와 같은 두족류를 모두 잡아먹는 육식성 물고기에 속한다.
미흑점상어는 태평양, 대서양, 인도양에 골고루 서식하며 홍해, 지중해, 페르시아만 등에서도 서식하는 어종이기도 하다. 전세계의 따듯한 온대와 열대의 바다에 두루두루 서식하며 수심 0~500M의 대륙붕과 대륙사면으로 이뤄진 표해수층과 중심해에서 주로 서식하는 어종이지만 때로는 얕은 연안에서 발견되기도 한다. 미흑점상어는 식용으로도 쓰이는 어종인데 식용으로 사용할 시에는 지느러미와 간, 육질이 식용으로 이용이 된다. 단 흉상어과에 속하는 어종답게 이빨이 날카로운만큼 살아있는 개체를 다룰 때는 주의가 요망되며 그래서 두꺼운 장갑과 보호 장비를 갖추고 손질을 하는 것이 좋다. 미흑점상어를 식용으로 먹을 때는 주로 샥스핀, 스쿠알렌, 회, 구이로 가장 많이 먹는다.